INTRODUCTION
Sarcopenia is a debilitating and progressive condition that is characterized by the increasingly severe loss of muscle mass and associated strength. In general, these reductions in muscle mass begin in individuals over 40 years of age and progress by an estimated 3-8% every 10 years1. In individuals between the ages of 60 and 70 years, sarcopenia prevalence is thought to range from 5-13%, with these rates rising with further advances in age2. Importantly, sarcopenia is often related to adverse outcomes, including osteoporosis, falls, a dependent lifestyle, and an elevated risk of mortality3. As such, efforts to identify and treat sarcopenia represent a major challenge to global public health.
A number of comorbid conditions have the potential to accelerate the physiological progression of sarcopenia4. In particular, chronic heart failure (CHF) is a highly prevalent condition among older adults and is the result of progressive contractile or diastolic cardiac dysfunction and deterioration with decreased cardiac output5. Many prior studies have identified a close relationship between CHF and sarcopenia, with the former having been suggested to contribute to the more rapid and severe deterioration of skeletal and cardiac muscle6-8. Consistently, sarcopenia rates are higher among older adults diagnosed with CHF compared to healthy older adults, and sarcopenic individuals have the potential to exhibit a worse heart failure prognosis9. The onset of sarcopenia is also often earlier in individuals with CHF regardless of their cardiac output, and the proper diagnosis and monitoring of these patients at an early time point are essential to guide their treatment6. At present, however, reliable biomarkers capable of detecting sarcopenia in patients diagnosed with CHF are lacking.
MicroRNAs (miRNAs) are 21-23 nucleotide RNAs that lack coding potential but are capable of regulating the translation and stability of specific mRNA targets, thereby influencing a diverse array of pathological and physiological processes10. Many studies have outlined clear roles for specific miRNAs in sarcopenia and other diseases of the skeletal muscle. Importantly, skeletal muscle-derived miRNAs can be readily detected in circulation, underscoring their potential diagnostic utility11,12. In addition, individuals diagnosed with CHF frequently exhibit perturbations in their plasma miRNA profiles13. Of note, miR-1-3p has emerged as a promising candidate biomarker for the monitoring of muscular health as it may be dysregulated in individuals with CHF and/or sarcopenia14,15. However, further research is necessary to explain the relationship between circulating miR-1-3p levels and sarcopenia incidence/severity in CHF patient populations. Moreover, while the Akt/mTOR pathway has been shown to influence circulating miRNA profiles and overall disease status16, the specific relationship between such signaling and miR-1-3p levels in individuals with sarcopenia has not been reported.
Here, we explored, whether circulating miR-1-3p levels were associated with sarcopenic indices in individuals with CHF and evaluated the diagnostic utility of this miRNA as a biomarker for sarcopenia in a CHF patient cohort.
METHODS
Study population
This study was a cross-sectional observational study of the electronic medical records of individuals admitted to the Department of Geriatrics, People's Hospital of Xinjiang Uygur Autonomous Region, Urumqi, China, from January 1, 2020, to August 31, 2021. The primary patients admitted to this unit are older adults with chronic respiratory and cardiovascular diseases. To be eligible for inclusion in this study, patients had to meet the following criteria: ad to meet the following criteriatients admitted to this unit are older adults with chlar ejection fraction < 50% as measured by echocardiography; non-ischemic or ischemic etiologies; compensated heart failure with appropriate pharmacological treatment for a minimum of 3 months before the study period; and New York Heart Association Class II-IV.
Patients were excluded from this study if they had undergone major surgery, heart transplantation, pacemaker implantation, or suffered from autonomic diabetic neuropathy, unstable angina, myocardial infarction with percutaneous coronary intervention, ongoing infections, a history of cancer, stroke, or prolonged bed rest within 8 weeks before clinical evaluation.
The Ethics Committee of the People's Hospital of Xinjiang Uygur Autonomous Region (Urumqi, China) approved the study, which was conducted as per the Declaration of Helsinki. All patients provided written informed consent.
Handgrip strength (HGS) and body composition analyses
A digital handgrip dynamometer (CAMRY, CA, USA) was used to measure patient HGS. Briefly, individuals were directed to squeeze smoothly the dynamometer with maximal strength while seated; this was repeated 3 times per hand, with 60 s between tests. The maximum value was recorded for subsequent analysis. A bioelectrical impedance analysis scale (RENPHO, Dubai, UAE) was used to calculate appendicular skeletal muscle mass (ASM) and fat mass, with the ASM index (ASMI) being calculated by dividing ASM by the square of the participant's height2.
Physical performance measurements
Short physical performance battery (SPPB) scores were used for analyses of physical performance. For this test, patients are evaluated to assess their balance, walking speed over 4 m, and performance on a chair-stand test. Results for each test were scored from 0-4, with lower scores corresponding to worse performance. The sums of these three scores were added together for a final score of 0-12, as detailed in prior studies17.
Walking speed was determined by measuring patient gait speed over a 4 m distance (in m/s), with scores being assigned as follows based on the quartiles for the sample population: ≤ 0.38 m/s, 1; 0.39-0.57 m/s, 2; 0.58-0.76 m/s, 3; and ≥ 0.77m/s, 4.
For the chair-stand test, patients were directed to stand from a chair while keeping their arms folded over their chest as quickly as possible 5 times in a row, with the overall time required to complete the task being measured. Quartile scores for this patient population were as follows: ≥ 17.0 s, 1; 14.1-16.9 s, 2; 11.9-14.0 s, 3; and ≤ 11.8 s, 4.
For the balance test, patients were directed to stand in three increasingly difficult positions: side-by-side, semi-tandem, and tandem positions. They were told to hold each of these positions for 10 s. A score of 1 was assigned if they held the side-by-side position for 10 s but could not hold the semi-tandem position for 10 s; a score of 2 was assigned if they held the semi-tandem position for 10 s but could not hold the tandem position for 10 s; a score of 3 was assigned if they held the tandem position for 3-9 s; and a score of 4 was assigned if they held the tandem position for 10 s.
Quantification of RNA using real time-PCR
The TRI reagent was used to extract total RNA from 2 mL of whole blood samples, after which an iScriptTM cDNA Synthesis kit (Bio-Rad, CA, USA) was used to prepare cDNA from 1 µg of total RNA per sample as detailed previously17. Then, qPCR analyses were conducted using 2.5 ng cDNA samples amplified using the Fast SYBR Green master mix (Applied Biosystems, NY, USA). Thermocycler settings were as follows: 94°C for 30 s, 55°C for 30 s, and 72°C for 90 s for 40 total cycles. The normalization of miRNA levels was performed with U6, and the normalization of mRNA levels was performed with GAPDH, as previously described. Primers' sequences were as follows:
miR-1-3p (MIMAT0000416), 5'-CGCAGTGGAATGTAAAGAAG-3';
U6, 5'-CTCGCTTCGGCAGCACA-3';
Akt (NM_001014431.2), F:5'-TCTATGGCGCTGAGATTGTG-3',
R:5'-CTTAATGTGCCCGTCCTTGT-3';
mTOR (NM_001386500.1), F:5'-AGCTCTTCGGCCTGGTTAAC-3',
R:5'- CTTCTCCCTGTAGTCCCGGA-3';
GAPDH (NM_001256799.3), F:5'-TGTTGCCATCAATGACCCCTT-3',
R:5'-CTCCACGACGTACTCAGCG-3'
Statistical analyses
SPSS 21.0 (SPSS Inc., IL, USA) was used for all statistical testing. Anthropometric data were normally distributed and given as means with standard deviations. Data were compared through ANOVAs, while linear regression analyses were used to explore the relationship between miR-1-3p expression and different sarcopenic indices. Two-sample t-tests were used when comparing SPPB scores between groups. The diagnostic sensitivity and specificity values for miR-1-3p were examined using receiver operating characteristic curves. A two-tailed p < 0.05 was the threshold of significance.
RESULTS
Characteristics of the participants
The basic characteristics of the study population are summarized in Table 1. A total of 80 CHF patients were included in the present study, encompassing 40 sarcopenia patients (mean age 74.85 ± 1.047 years) and 40 patients without sarcopenia (mean age 77.8 ± 1.073 years). Overall, subjects in the sarcopenia group had lower ASMI, HGS, and walking speed (all p < 0.05). These patients also performed poorly on SPPB scores compared with the patients without sarcopenia (p < 0.05). Moreover, the sarcopenia patients were associated with higher total cholesterol and low-density lipoprotein cholesterol (all p < 0.05). There were no differences between the groups with respect to age, gender, body mass index, triglycerides, and hemoglobin A1C.
Table 1. Characteristics of the participants
| Variables | Patients with sarcopenia (n = 40) | Patients without sarcopenia (n = 40) | P value |
|---|---|---|---|
| Age at baseline (years) | 74.85 ± 1.047 | 77.8 ± 1.073 | 0.053 |
| Male | 20 (50%) | 25 (62.5) | 0.368 |
| Body composition | |||
| BMI (kg/m2) | 24.33 ± 0.646 | 24.93 ± 0.825 | 0.568 |
| ASMI (kg/m2) | 6.26 ± 0.174 | 6.93 ± 0.270 | 0.04 |
| Physical capacity | |||
| HGS (kg) | 18.34 ± 0.681 | 20.59 ± 0.753 | 0.03 |
| 4-meter walking speed (m/s) | 0.91 ± 0.15 | 1.23 ± 0.18 | < 0.001 |
| SPPB score | |||
| 6 ≤ x < 8 | 8 (20%) | 3 (7.5%) | 0.025 |
| 8 ≤ x < 10 | 26 (65%) | 21 (52.5%) | |
| 10 ≤ x ≤ 12 | 6 (15%) | 16 (40%) | |
| Plasma profile | |||
| Total cholesterol | 4.34± 0.165 | 3.71 ± 0.152 | 0.006 |
| LDL-C (mg/dL) | 2.63 ± 0.143 | 2.02 ± 0.106 | 0.001 |
| Triglycerides (mg/dL) | 1.35 ± 0.071 | 1.19 ± 0.097 | 0.185 |
| HbA1c (%) | 6.93 ± 0.447 | 6.82 ± 0.312 | 0.837 |
Continuous data are expressed as mean ± SD and categorical data are expressed as n (%).
Levels of circulating miRs and the Akt/mTOR signaling
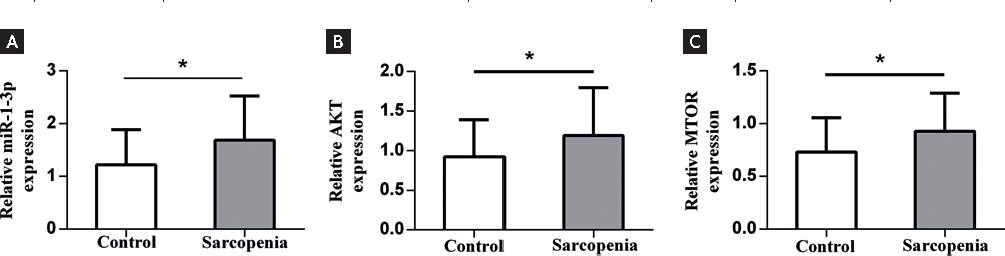
We next measured the levels of selected circulating miR-1-3p expression, AKT, and mTOR in the patients with sarcopenia and control subjects without sarcopenia. Patients with sarcopenia showed a significantly higher level of miR-1-3p (1.69 ± 0.132) than those subjects without sarcopenia (1.22 ± 0.106, p < 0.05). On the other hand, when compared with the control group without sarcopenia, the sarcopenia patients had higher levels of AKT (1.19 ± 0.095 vs. 0.92 ± 0.074, p = 0.029) and mTOR (0.9276 ± 0.057 vs. 0.73 ± 0.052, p = 0.012, Fig. 1).
Correlation of miR-1-3p with sarcopenia indexes and Akt/mTOR signaling
The Asian Working Group on Sarcopenia in Older People characterizes sarcopenia with low muscle strength and mass2. We used ASMI and HGS as the indexes for the detection of sarcopenia. miR-1-3p showed the strongest correlation with ASMI in all study subjects adjusted for age (r2 = 0.210, p < 0.001). We also found significant correlations of miR-1-3p with HGS in all study subjects adjusted for age (r2 = 0.220, p < 0.001). On the other hand, there was a relationship between the miR-1-3p and Akt/mTOR in all study subjects adjusted for age (Fig. 2) (all p < 0.001).
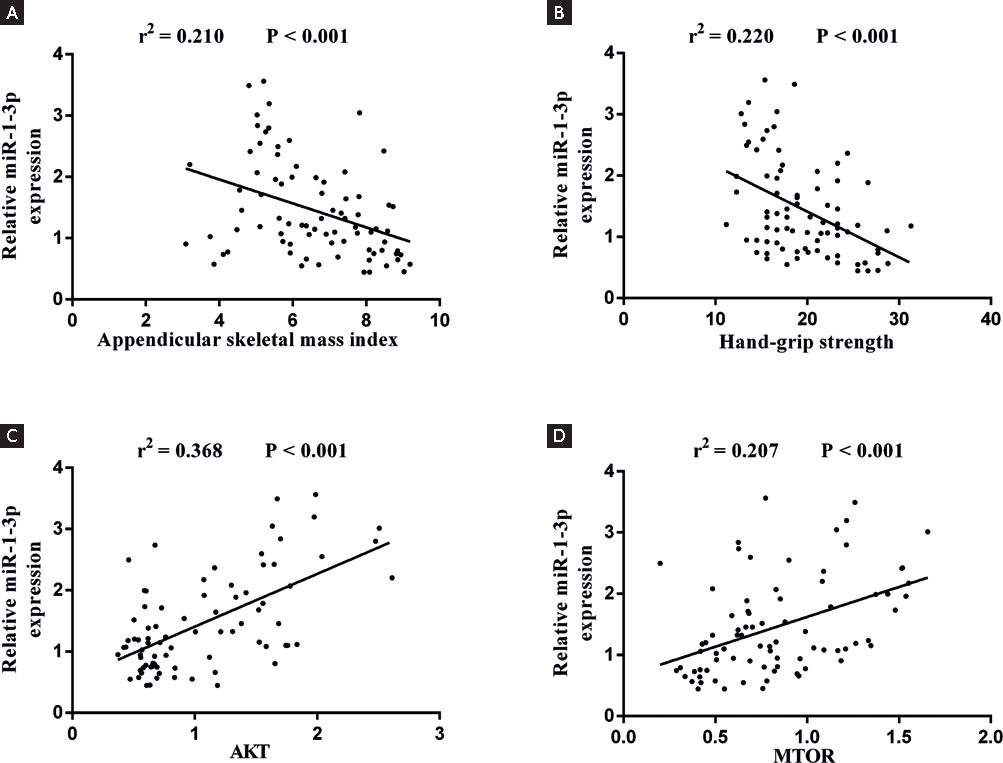
Figure 2. A: Linear regression analysis of the relationships of plasma miR-1-3p expression with appendicular skeletal mass index. B: hand-grip strength. C: AKT. D: mTOR in all study subjects adjust for age.
Circulating miRs accurately identified accelerated sarcopenia in CHF
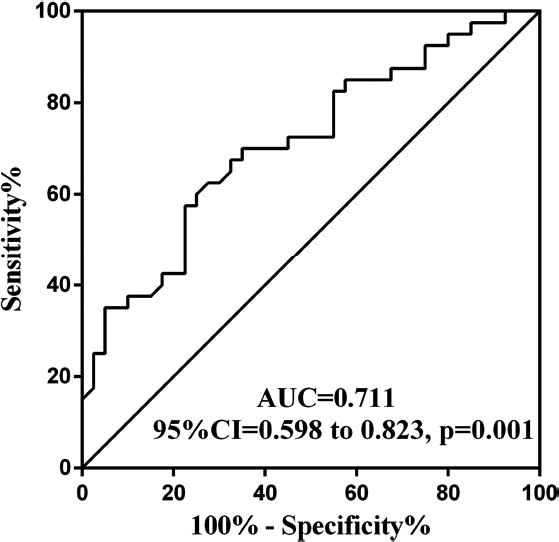
We next tested the diagnostic potentials of circulating miR-1-3p in CHF patients with sarcopenia, using receiver operative characteristic (ROC) curves (Fig. 3). The area under the ROC curve for miR-1-3p was 0.711. The cutoff value for the prediction of sarcopenia was 1.01. A miR-1-3p > 1.01 predicted sarcopenia with 75.0% sensitivity and 62.5% specificity. It indicated that the small molecule had a certain diagnostic value.
DISCUSSION
In this study, we found that sarcopenic individuals with CHF exhibited higher plasma miR-1-3p levels relative to CHF patients without sarcopenia. Moreover, plasma miR-1-3p levels were strongly correlated with HGS and ASMI in these patients, and a significant link between miR-1-3p expression and Akt/mTOR pathway activation was observed. In addition, miR-1-3p was identified as a robust, sensitive, and specific biomarker for sarcopenia among individuals with CHF.
Sarcopenia is a complex syndrome that affects older individuals. In addition, many non-age-related conditions can contribute to weakness and overall muscle atrophy, highlighting the sensitivity of skeletal muscle to systemic conditions18. CHF patients exhibit higher sarcopenia incidence rates as compared to age-matched healthy older adults, and they exhibit a more rapid rate of muscle deterioration, contributing to additional reductions in cardiac functionality19. A number of mechanistic factors have been reported to contribute to these reductions in muscle mass in CHF patients, including malnutrition, hormonal changes, malabsorption, and increases in oxidative stress and inflammatory activity. These factors can, either alone or together, contribute to reductions in muscle protein synthesis and increased rates of muscle protein degradation7. Sarcopenia is highly prevalent among CHF patients, contributing to a poorer patient prognosis, and underscoring the need to identify viable biomarkers of sarcopenia among individuals with CHF.
Many miRNAs have been shown to exhibit weak, but significant correlations with sarcopenic indice, consistent with a relationship between plasma miRNA profiles and muscle degradation12. In this study, we found miR-1-3p to be most strongly correlated with HGS and ASMI and were identified as a specific marker for sarcopenia in individuals with CHF. These results are in line with data from Teodori et al.15, who demonstrated that miR-1-3p is mainly generated by skeletal muscle and regulates the proliferation and differentiation of muscle cells. However, many studies of this miRNA have primarily focused on healthy older adults, with few specific analyses of CHD patients having been conducted to date. In many reports, miR-1-3p has been shown to be a promising biomarker related to the pathogenesis of heart failure, and strong links have been identified between miR-1-3p levels in the plasma and CK levels, suggesting that muscle damage plays at least some role in shaping plasma miR-1-3p expression profiles20,21. CHF contributes to damage to the skeletal muscle and associated cellular necrosis, potentially leading to passive miR-1-3p release into systemic circulation. Both CHF and sarcopenia may influence miR-1-3p release and may, in turn, be functionally regulated by this miRNA. Here, we found plasma miR-1-3p levels to be higher among individuals with both CHF and sarcopenia as compared to levels in non-sarcopenic CHF patients. As such, miR-1-3p offers promise as a biomarker of sarcopenia in CHF patient populations.
In the present study, we did not conduct any functional analyses of miR-1-3p target genes. The Akt/mTOR pathway plays a central role in the regulation of cellular proliferation and survival in both physiological and pathological contexts22. Akt/mTOR signal pathway plays important regulatory roles for autophagy in skeletal muscle. Autophagy quality control in skeletal muscle is closely related to sarcopenia, and dysregulated autophagy quality control leads to sarcopenia in naturally or rapidly aged mice23. A study showed that a defective signaling through Akt/mTOR in response to insulin is a central and common mechanism of insulin resistance in diabetic sarcopenia24. miR-1-3p can suppress colorectal cell proliferation through targeting this Akt/mTOR axis25, but no corresponding studies have been conducted in patients with CHF or sarcopenia. Here, we found miR-1-3p expression to be highly correlated with the Akt/mTOR signaling pathway, with components of this pathway being expressed at high levels in sarcopenic individuals. In animals, the inhibition of mTOR using rapamycin induced reductions in type I fiber cross-sectional area in heart failure model mice, impairing aerobic exercise training anti-atrophic effects on aerobic exercise training soleus26. These data highlight the role of mTOR as a mediator of aerobic exercise training soleus attenuation of muscle wasting in the context of heart failure. Aging muscle tissue commonly exhibits neuromuscular junction (NMJ) instability that is believed to contribute to the pathogenesis of sarcopenia. Other studies have suggested that increased mTORC1 signaling with age contributes to NMJ deterioration and sarcopenic development27. Together, these results support the potential value of miR-1-3p as a biomarker for physical capacity and muscle health in older CHF patients with sarcopenia that may function through the Akt/mTOR pathway.
There are certain limitations to this analysis. For one, this was a cross-sectional study, and we were thus unable to draw causative inferences or to assess longitudinal outcomes. In addition, our sample size was limited. Further large-scale longitudinal studies will be needed to validate these results, and additional research will also be required to clarify the mechanisms linking miR-1-3p expression to sarcopenia in individuals with CHF.
Here, we found that plasma miR-1-3p levels offer potential value as a novel biomarker for physical capacity and overall skeletal muscle health in individuals diagnosed with CHF, possibly due to a mechanistic link between this miRNA and Akt/mTOR pathway activity. As such, monitoring plasma miR-1-3p levels may offer value as a means of diagnosing sarcopenia or guiding patient management/stratification efforts among individuals with CHF. However, further work will be necessary to clarify the functional importance of miR-1-3p as a modulator of sarcopenic incidence in CHF patient populations.











 nueva página del texto (beta)
nueva página del texto (beta)