INTRODUCTION
Hypertension (HTN), which is increasing in prevalence globally and is an important risk factor for mortality, may be diagnosed late due to its symptomless nature1. Therefore, most patients with HTN may have asymptomatic organ damage (AOD) at their first hospital admission. Atherosclerosis due to cardiac and vascular tissue damage induced by high blood pressure (BP) causes AOD2. AOD reaching critical levels result in irreversible organ dysfunction3. Thus, the detection of AOD and the determination of a treatment strategy at the first hospital admission of hypertensive patients are of prognostic importance.
Damage to endothelial tissue due to high BP is associated with the inflammatory response and contributes significantly to hypertensive organ damage4,5. High levels of reactive oxygen species (ROS) have been detected in the dendritic cells of patients with HTN and animal models of HTN6. Increased ROS levels cause activation of innate and acquired immune cells. T-cells have been shown to migrate to the kidneys and vascular tree in animal models of hypertension. This is hypothesized to contribute to endothelial cell dysfunction and kidney damage7-9. In AOD, it plays a role in the innate and adaptive immune systems as well as HTN-related hemodynamic disorders10. In atherosclerosis, which plays a role in the pathogenesis of HTN, platelet and leukocyte infiltration are the driving force of the atherosclerotic lesions, dependent on inflammation, while neutrophils are the first line of defense11,12. In contrast, platelets play a role in regulating the effector functions of neutrophils and macrophages13. These mechanisms have an important role in the inflammatory response mediated by the innate or adaptive immune system in hypertensive organ damage.
Low-grade inflammation is known to facilitate the development of hypertensive organ damage14. The systemic immune-inflammation index (SII) is a new inflammatory index based on circulating immune-inflammatory cells15. Therefore, we hypothesized that the SII could be a simple, economic, and feasible screening tool to predict the presence and severity of AOD. In this study, we aimed to examine the relationship between the SII and AOD in patients with newly diagnosed treatment-naive HTN.
METHODS
This study had a retrospective design and was performed at the Kirikkale High Specialization Hospital's Cardiology Clinic from January 2021 to December 2021. The study was approved by the Local Ethics Committee (Decision No. 2022.03.15), and written informed consent was obtained from all patients.
Study population
A total of 500 participants (≥ 18 years) were enrolled in the study, including 250 patients who were newly diagnosed with treatment-naive HTN and 250 healthy volunteers in the Cardiology Clinic of Kirikkale Yuksek Ihtisas Hospital. Patients who presented for check-ups and did not have any chronic diseases or drug use were selected as the control group.
Previously known or documented diagnosis of primary or secondary HTN, smoking and alcohol use, obesity, any additional diseases (diabetes mellitus, rheumatic diseases, documented coronary artery disease, malignancy, active or chronic infection, acute or chronic kidney disease, peripheral arterial disease, cerebrovascular disease, heart failure, and liver diseases), nephrotic-level proteinuria, and the use of antioxidant substances or lipid drugs were exclusion criteria.
Study protocol
HTN was defined as systolic BP (SBP) of ≥ 140 mmHg and diastolic BP (DBP) of ≥ 90 mmHg. Microalbuminuria of > 30 mg/day or proteinuria of > 150 mg/day left ventricular mass index (LVMI) of > 95 g/m2 in women and > 115 g/m2 in men, and carotid intima-media thickness (CIMT) of > 0.9 mm or the presence of plaque in the carotid were evaluated as AOD indicators16. Patients with any of these indicators were evaluated as AOD (+). In addition, AOD grade was classified as follows: Grade I - One organ involved, Grade II - Two organs involved, Grade III - Three organs involved, and Grade IV - Four organs involved17. Body mass index (BMI) was calculated as follows: Body weight (kg)/height2 (m2). The SII was calculated with the following formula: Neutrophil count × Platelet count/Lymphocyte count.
Biochemical parameters
Fasting laboratory parameters were measured at the time of admission to the hospital. Lipid parameters (by enzymatic colorimetric methods), proteinuria, and microalbuminuria in 24 h (by turbidimetric methods) were evaluated with a Hitachi Modular P800 autoanalyzer (Roche Diagnostic Corp., Indianapolis, IN, USA). Erythrocytes and platelets were measured by impedance (resistance) method, leukocytes were measured by optical laser scattering (light scattering), and other complete blood count parameters were measured with a Sysmex XE 2100 hematology analyzer (Roche Diagnostic Corp., Indianapolis, IN, USA). Hemoglobin was measured photometrically.
BP measurement
Following 5 min of resting after hospital admission, all patients underwent three different BP measurements at 5-min intervals and their average was taken. BP measurements were made with an Omron M3 automatic sphygmomanometer (Omron Healthcare, Tokyo, Japan). The diagnosis of hypertension was evaluated according to the 2018 ESC criteria18.
Echocardiographic examination
All echocardiographic examinations were performed by a cardiologist blinded to the study with an echocardiography device (2.5 MHz transducer, Vivid 7, GE-Vingmed Ultrasound AS, Horten, Norway). LVM was computed with the Devereux formula through 2D echocardiographic measurements. The Devereux formula of LVM = 1.04 × ([IVSTd + PWTd + LVIDd]3 – [LVIDd]3) – 13.6 was used and was indexed to body surface area (IVSTd: Interventricular septum thickness in diastole; PWT: Posterior wall thickness in diastole; and LVIDd: Left ventricular internal diameter in diastole)19. LVM index (LVMI) of > 95 g/m2 in women and > 115 g/m2 in men was considered an indicator of the left ventricular hypertrophy.
Carotid ultrasonography
CIMT was evaluated with the patient in a supine position with both hands under the head. CIMT values were measured with a high-resolution B-mode device (Logiq 7, GE Med Inc., Chicago, IL, USA) by a radiologist blinded to the clinical status of the patients. All measurements were made of the right and left main carotid arteries using a linear probe with an automatic system. Measurements were performed at three points: the right carotid artery branches from the brachiocephalic trunk, the left carotid arteries from the aorta at 2 cm away, and the bifurcation of the internal carotid arteries. Longitudinal measurements were performed from distances of media-adventitia echogenicity and vessel lumen echogenicity. CIMT was calculated by taking the average of three measurements made for each carotid artery.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 26 for Windows (IBM Corp., Armonk, NY, USA). The extent to which the data followed a normal distribution was evaluated using the Kolmogorov–Smirnov test. Numerical variables with and without normal distribution were plotted as mean ± standard deviation and median (25th and 75th interquartile range [IQR]), respectively. Categorical variables were indicated as numerical and percentile values. Correlations between numerical parameters were analyzed through Pearson and Spearman correlation analysis. Chi-square, Yates correction, and Fisher exact tests were used for the comparison of categorical data. Student's t-test or Mann–Whitney U-test was used for the comparison of numerical variables between the two groups according to the distribution of normality. ANOVA (post hoc: Bonferroni test) or the Kruskal–Wallis H test (post hoc: Dunn test) was used for the comparison of numerical variables based on AOD grade according to the distribution of normality. Stepwise multivariable logistic regression analysis was used to predict AOD. Diagnostic performance assessment of the SII was performed by ROC curve analysis, and area under the curve (AUC), standard error (SE), and positive (+PV) and negative (−PV) predictive values are presented. The optimal threshold value of the SII in predicting AOD was determined by the Youden index method. Values of p < 0.05 were considered to be significant in statistical analysis.
RESULTS
The study included 250 patients with newly diagnosed HTN (mean age 50.8 ± 9.4, female ratio 64.8%) and 250 healthy controls (mean age 51.6 ± 11.4, female ratio 66.0%). The distributions of gender, age, and BMI were similar for the control and newly diagnosed HTN groups (p > 0.05). The median neutrophil and mean platelet levels in the newly diagnosed HTN group were higher compared with the control group, while mean lymphocyte levels were lower. The median SII was higher in the newly diagnosed HTN group than in the control group (508.1 vs. 320.2, p < 0.001). The levels of AOD indicators were higher in the newly diagnosed HTN.
In patients with newly diagnosed HTN, the SII levels positively correlated with proteinuria levels (r = 0.421; p < 0.001), the urinary albumin levels (r = 0.416; p < 0.001), the LVMI levels (r = 0.448; p < 0.001), and with the CIMT values (r = 0.534; p < 0.001) (Table 1). In addition, SII levels positively correlated with C-reactive protein (CRP) levels (r = 0.352; p < 0.001).
Table 1. Factors associated with asymptomatic organ damage in patients with newly diagnosed treatment-naive hypertension
| Variables | CIMT | LVMI | Microalbuminuria | Proteinuria | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Age | 0.211 | 0.001* | 0.241 | 0.026* | 0.019 | 0.762 | 0.008 | 0.901 |
| BMI | 0.094 | 0.139 | 0.037 | 0.562 | 0.081 | 0.200 | 0.063 | 0.110 |
| SBP | 0.024 | 0.708 | 0.052 | 0.414 | 0.027 | 0.676 | 0.030 | 0.957 |
| DBP | 0.085 | 0.181 | 0.086 | 0.177 | 0.050 | 0.970 | 0.007 | 0.907 |
| FBG | 0.102 | 0.107 | 0.010 | 0.877 | 0.065 | 0.311 | 0.066 | 0.298 |
| WBC | 0.090 | 0.154 | 0.096 | 0.131 | 0.256 | 0.015* | 0.030 | 0.642 |
| Neutrophil | 0.416 | < 0.001* | 0.420 | < 0.001* | 0.332 | < 0.001* | 0.326 | < 0.001* |
| Platelet | 0.309 | 0.001* | 0.118 | 0.063 | 0.292 | < 0.001* | 0.270 | < 0.001* |
| Lymphocyte | 0.050 | 0.432 | 0.040 | 0.528 | 0.027 | 0.666 | 0.010 | 0.880 |
| Hemoglobin | −0.092 | 0.147 | 0.004 | 0.948 | 0.008 | 0.904 | −0.047 | 0.463 |
| Total cholesterol | 0.085 | 0.186 | −0.076 | 0.240 | −0.009 | 0.884 | 0.008 | 0.906 |
| LDL | 0.078 | 0.225 | −0.041 | 0.524 | −0.008 | 0.903 | 0.005 | 0.940 |
| HDL | −0.051 | 0.432 | 0.020 | 0.752 | −0.002 | 0.975 | 0.058 | 0.368 |
| Triglyceride | 0.245 | 0.022* | −0.116 | 0.070 | 0.010 | 0.880 | −0.007 | 0.918 |
| Albumin | −0.034 | 0.595 | 0.048 | 0.456 | −0.002 | 0.979 | 0.054 | 0.394 |
| CRP | 0.298 | 0.023* | 0.288 | 0.049* | 0.292 | 0.012* | 0.272 | 0.050* |
| SII | 0.534 | < 0.001* | 0.448 | < 0.001* | 0.416 | < 0.001* | 0.421 | < 0.001* |
| NLR | 0.406 | < 0.001* | 0.355 | < 0.001* | 0.272 | < 0.001* | 0.311 | < 0.001* |
| PLR | 0.343 | < 0.001* | 0.302 | < 0.001* | 0.265 | < 0.001* | 0.268 | < 0.001* |
BMI: body mass index; CIMT: carotid intima media thickness; CRP: C-reactive protein; DBP: diastolic blood pressure; FBG: fasting blood glucose; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LVMI: left ventricular mass index; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; SBP: systolic blood pressure; SII: systemic immune-inflammation index; WBC: white blood cell.
*p < 0.05 was considered statistically significant.
AOD was detected in 56% of patients with newly diagnosed treatment-naive HTN. The distribution of demographic and laboratory findings according to the presence of AOD is shown in Table 2. The mean age was higher in AOD (+) group compared to AOD (-) and control groups (control: 50.8 ± 9.4 vs. AOD (-): 49.7 ± 11.7 vs. AOD (+): 53.1 ± 10.9; p = 0.011), while other demographic findings did not differ between the groups. The median SII levels were highest in the AOD (+) group, followed by the AOD (-) and control groups, respectively (control: 320.2 vs. AOD (-): 462.3 vs. AOD (+): 600.7; p < 0.011). The median CRP levels were higher in the AOD (+) group than in other groups, while it was higher in the AOD (-) group than in control groups (Table 2).
Table 2. Demographic and laboratory findings associated with asymptomatic organ damage in newly diagnosed treatment-naive hypertension
| Variables | Control (n = 250) | Newly diagnosed hypertension | p | |
|---|---|---|---|---|
| AOD (-) (n = 110) | AOD (+) (n = 140) | |||
| Age, years | 50.8 ± 9.4 | 49.7 ± 11.7 | 53.1 ± 10.9 | 0.011* |
| Female gender, n (%) | 162 (64.8) | 66 (60.0) | 99 (70.7) | 0.201 |
| BMI, kg/m2 | 27.3 ± 4.6 | 27.5 ± 4.7 | 27.7 ± 5.2 | 0.169 |
| SBP, mm Hg | 129.5 ± 17.3 | 158.5 ± 14.3 | 160.8 ± 12.1 | < 0.001* |
| DBP, mm Hg | 78.8 ± 18.1 | 98.4 ± 9.4 | 99.7 ± 9.6 | < 0.001* |
| CIMT, mm | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.9 ± 0.2 | < 0.001* |
| LVMI, g/m2 | 74.2 ± 10.2 | 80.1 ± 12.7 | 98.1 ± 20.2 | < 0.001* |
| Microalbuminuria, g/24 h | 6.4 (3.6-14.4) | 8.0 (4.0-14.4) | 23 (8.8-44.2) | < 0.001* |
| Proteinuria, g/24 h | 56.3 (30.2-95.5) | 74.7 (55.3-102.4) | 134.9 (85.8-176.6) | < 0.001* |
| FBG, mg/dL | 95.9 ± 13.7 | 99.0 ± 11.9 | 96.9 ± 14.5 | 0.161 |
| WBC, × 109/L | 7.1 ± 1.6 | 7.2 ± 2.1 | 7.5 ± 2.2 | 0.114 |
| Neutrophil, × 109/L | 3.1 (2.8-3.5) | 3.2 (2.8-4) | 4.5 (3.9-5.4) | < 0.001* |
| Platelet, × 109/L | 233.4 ± 55.9 | 274.7 ± 61.7 | 300.5 ± 62.6 | < 0.001* |
| Lymphocyte, × 109/L | 2.4 ± 0.8 | 2.2 ± 0.8 | 2.2 ± 0.7 | 0.002* |
| Hemoglobin, d/dL | 14.1 ± 1.8 | 14.2 ± 1.7 | 14.0 ± 1.6 | 0.265 |
| Total cholesterol, mg/dL | 195.3 ± 49.4 | 207.1 ± 43.4 | 203.4 ± 42.7 | 0.061 |
| LDL, mg/dL | 112 (89-142) | 121 (100-149) | 124 (95-146) | 0.235 |
| HDL, mg/dL | 51.1 ± 12.7 | 48.9 ± 13.8 | 50.4 ± 12.9 | 0.366 |
| Triglyceride, mg/dL | 109 (79-163) | 147.5 (108-188) | 134 (97-196) | 0.008* |
| Albumin, g/dL | 45.3 ± 5.8 | 46.1 ± 3.1 | 46.2 ± 3.4 | 0.113 |
| CRP, mg/dL | 0.2 (0-0.5) | 2 (1-3.8) | 3.6 (2.3-6.7) | < 0.001* |
| SII | 320.2 (253.7-373.9) | 462.3 (423.8-505.5) | 600.7 (484.9-777.2) | < 0.001* |
| NLR | 1.4 (1.1-1.7) | 1.7 (1.5-1.9) | 2.1 (1.7-2.6) | < 0.001* |
| PLR | 94.0 (76.8-137.4) | 134.5 (101.9-161.4) | 139.5 (118.8-169.2) | < 0.001* |
AOD: asymptomatic organ damage; BMI: body mass index; CIMT: carotid intima media thickness; CRP: C-reactive protein; DBP: diastolic blood pressure; FBG: fasting blood glucose; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LVMI: left ventricular mass index; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; SBP: systolic blood pressure; SII: systemic immune-inflammation index; WBC: white blood cell.
*p < 0.05 was considered statistically significant. Bold characters show the difference between groups.
The frequency of AOD severity according to the number of organs involved in newly diagnosed HTN patients was as follows: Grade I in 26% (n = 65), Grade II in 16% (n = 40), Grade III in 7% (n = 17), and Grade IV in 7% (n = 18). The median SII levels increased as the AOD severity increased from Grade I to IV (Table 3).
Table 3. Demographic and laboratory findings associated with grade of asymptomatic organ damage in newly diagnosed treatment-naive hypertension
| Variables | AOD (-) (n = 110) | AOD (+) | p | ||
|---|---|---|---|---|---|
| Grade I (n = 65) | Grade II (n = 40) | Grade III-IV (n = 35) | |||
| Age, years | 49.7 ± 11.7 | 51.3 ± 10.5 | 51.8 ± 11.7 | 55.4 ± 11.2 | 0.028* |
| Female gender, n (%) | 66 (60.0) | 40 (61.5) | 30 (75.0) | 29 (82.9) | 0.040* |
| BMI, kg/m2 | 27.5 ± 4.7 | 28.0 ± 5.0 | 28.8 ± 5.4 | 28.8 ± 5.6 | 0.355 |
| SBP, mm Hg | 158.5 ± 14.3 | 157.2 ± 8.7 | 162.6 ± 16.2 | 161.4 ± 11.2 | 0.103 |
| DBP, mm Hg | 98.4 ± 9.4 | 97.8 ± 5.9 | 99.8 ± 11.3 | 99.1 ± 6.3 | 0.781 |
| CIMT, mm | 0.7 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | < 0.001* |
| LVMI, g/m2 | 80.1 ± 12.7 | 88.8 ± 16.3 | 97.3 ± 16.3 | 116.1 ± 19 | < 0.001* |
| Microalbuminuria, g/24 h | 8.0 (4.0-14.4) | 9.2 (5.2-23.0) | 22.6 (12.6-50.8) | 55.2 (38-78.5) | < 0.001* |
| Proteinuria, g/24 h | 74.7 (55.3-102.4) | 98 (66.2-135.6) | 137.5 (95.9-195.4) | 173 (155-234.8) | < 0.001* |
| FBG, mg/dL | 99.0 ± 11.9 | 96.9 ± 13.8 | 96.8 ± 12.7 | 96.9 ± 17.8 | 0.674 |
| WBC, × 109/L | 7.2 ± 2.1 | 6.9 (6.3-7.8) | 7.5 (6.3-8.3) | 7.8 (6.8-9.9) | 0.060 |
| Neutrophil, × 109/L | 3.2 (2.8-4) | 4 (3.6-4.6) | 4.6 (4.1-5.3) | 5.7 (4.6-7.4) | < 0.001* |
| Platelet, × 109/L | 274.7 ± 61.7 | 280.8 ± 53.5 | 301.2 ± 59.4 | 336.2 ± 67.4 | < 0.001* |
| Lymphocyte, × 109/L | 2.2 ± 0.8 | 2.4 ± 0.8 | 2.2 ± 0.5 | 2.1 ± 0.7 | 0.031* |
| Hemoglobin, d/dL | 14.2 ± 1.7 | 14.1 ± 1.4 | 13.7 ± 1.6 | 13.8 ± 1.6 | 0.175 |
| Total cholesterol, mg/dL | 207.1 ± 43.4 | 202.5 ± 39.1 | 206.5 ± 52.7 | 201.2 ± 36.6 | 0.858 |
| LDL, mg/dL | 121 (100-149) | 124 (99-139) | 119 (88.5-150.5) | 129.5 (98.5-146) | 0.898 |
| HDL, mg/dL | 48.9 ± 13.8 | 48.7 ± 12.6 | 51.2 ± 12.4 | 52.7 ± 14.1 | 0.427 |
| Triglyceride, mg/dL | 147.5 (108-188) | 138 (97-202) | 141.5 (106.5-209) | 118 (89.5-136) | 0.097 |
| Albumin, g/dL | 46.1 ± 3.1 | 46.4 ± 2.8 | 46.4 ± 2.3 | 45.5 ± 5 | 0.573 |
| CRP, mg/dL | 2 (1-3.8) | 3.4 (2-5.9) | 3.7 (2.6-6.2) | 4.4 (1.8-10.4) | 0.008* |
| SII | 462.3 (423.8-505.5) | 498.3 (432.7-567.5) | 670.8 (576.9-759.8) | 931 (810-1141.4) | < 0.001* |
| NLR | 1.7 (1.5-1.9) | 1.8 (1.6-2.1) | 2.2 (1.9-2.5) | 3.0 (2.3-3.7) | < 0.001* |
| PLR | 134.5 (101.9-161.4) | 136.7 (122.8-166.0) | 138.4 (118.9-165.7) | 165.9 (133.3-193.3) | < 0.001* |
AOD: asymptomatic organ damage; BMI: body mass index; CIMT: carotid intima media thickness; CRP: C-reactive protein; DBP: diastolic blood pressure; FBG: fasting blood glucose; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LVMI: left ventricular mass index; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; SBP: systolic blood pressure; SII: systemic immune-inflammation index; WBC: white blood cell.
*p < 0.05 was considered statistically significant. Bold characters show the difference between groups.
Multivariable regression models, in which variables associated with the presence and severity of AOD was included, showed that SII was an independent predictor of the presence and all grades of AOD (Table 4). The BP and CRP levels did not correlate with the presence and grades of AOD.
Table 4. Independent predictors of presence and severity of asymptomatic organ damage
| Variables | Multivariable Regression | |||
|---|---|---|---|---|
| OR | 95% CI | p | ||
| Lower | Upper | |||
| AOD (+) (ref: AOD (–)) | ||||
| Age | 1.03 | 1.01 | 1.06 | 0.045* |
| CIMT | 1.14 | 1.09 | 1.19 | < 0.001* |
| LVMI | 1.08 | 1.04 | 1.12 | < 0.001* |
| Microalbuminuria | 1.09 | 1.04 | 1.14 | < 0.001* |
| Proteinuria | 1.02 | 1.01 | 1.03 | < 0.001* |
| SII | 1.05 | 1.01 | 1.11 | 0.002* |
| Nagelkerke R2 = 0.754 | < 0.001* | |||
| Grade I (ref: AOD (–)) | ||||
| CIMT | 1.13 | 1.09 | 1.18 | < 0.001* |
| LVMI | 1.06 | 1.03 | 1.10 | < 0.001* |
| Proteinuria | 1.02 | 1.01 | 1.03 | < 0.001* |
| SII | 1.06 | 1.01 | 1.11 | 0.012* |
| Nagelkerke R2 = 0.653 | < 0.001* | |||
| Grade II (ref: Grade I) | ||||
| LVMI | 1.04 | 1.01 | 1.09 | 0.047* |
| Microalbuminuria | 1.06 | 1.02 | 1.10 | 0.002* |
| SII | 1.02 | 1.01 | 1.03 | < 0.001* |
| Nagelkerke R2 = 0.722 | < 0.001* | |||
| Grade III-IV (ref: Grade II) | ||||
| Age | 1.07 | 1.01 | 1.14 | 0.049* |
| CIMT | 1.09 | 1.01 | 1.18 | 0.032* |
| LVMI | 1.07 | 1.01 | 1.13 | 0.027* |
| Microalbuminuria | 1.09 | 1.04 | 1.15 | < 0.001* |
| SII | 1.02 | 1.01 | 1.03 | < 0.001* |
| Nagelkerke R2 = 0.788 | < 0.001* | |||
AOD: asymptomatic organ damage; CIMT: carotid intima media thickness; LVMI: left ventricular mass index; SII: systemic immune-inflammation index; OR: odds ratio; CI: confidence interval.
*p < 0.05 is considered significant for statistical analyses.
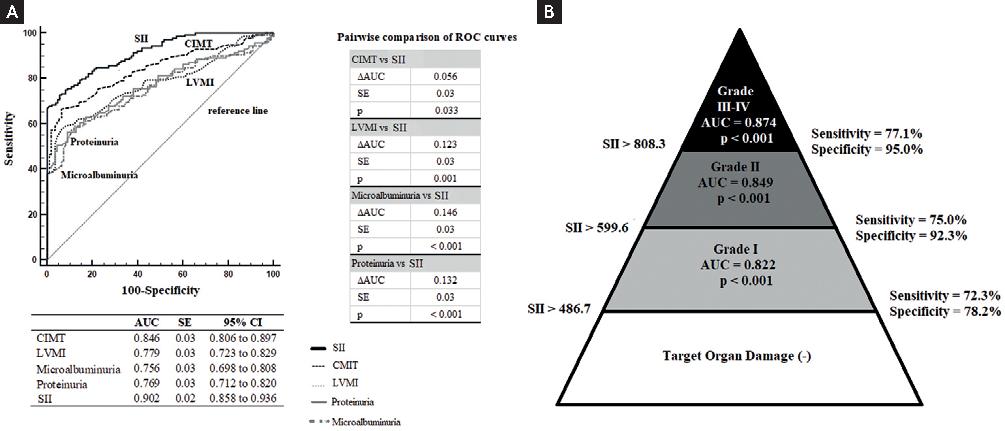
An SII level higher than 486.7 with 75.7% sensitivity and 90.0% specificity was found to be a predictor of AOD (AUC ± SE = 0.902 ± 0.02, +PV = 90.6%, –PV = 74.4%, p < 0.001). The SII had superior diagnostic discrimination compared to other AOD indicators in predicting the presence of AOD (Fig. 1A). Threshold values of SII in predicting the degree of AOD are shown in figure 1B. In addition, SII had superior diagnostic performance than neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) (SII vs. NLR: ∆AUC ± SE = 0.120 ± 0.04, p < 0.001; SII vs. NLR: ∆AUC ± SE = 0.184 ± 0.05, p < 0.001).
DISCUSSION
This study is the first to report that SII values were higher among newly diagnosed treatment-naive HTN patients compared to healthy controls. In patients with HTN, there was a positive correlation between SII values and all indicators of AOD, and SII values were higher among AOD (+) patients compared to AOD (–) patients. Threshold values showing a systemic increase in SII levels had high diagnostic performance in predicting the severity of AOD relative to the number of organs involved.
We detected the presence of AOD in most newly diagnosed treatment-naive HTN patients. Moreover, 14% of HTN patients had involvement of more than two organs. In another study conducted with newly diagnosed HTN patients, the prevalence of target organ damage was 60.7%, while the rate of involvement of more than two organs was 12%20. These findings indicated that the newly diagnosed HTN cohort was not aware of AOD at the time of their first visit to the hospital. As a result of the asymptomatic nature and late diagnosis of HTN, overt hypertensive organ damage may develop silently before it becomes clinically evident. In clinical practice, hypertensive organ damage is detected by CIMT, LVMI, urinary microalbuminuria, and urinary protein excretion, which requires further research and expertise21. Therefore, recent studies have focused on simpler and more economical AOD indicators, particularly those derived from blood parameters, which can be easily applied in any hospital, because the most common laboratory data universally available at the time of admission to the hospital are blood parameters22. Considering the role of inflammation in HTN, the SII, which can be easily calculated from blood parameters, can be a prognostic screening tool for newly diagnosed HTN patients.
HTN is an atherosclerotic and chronic inflammatory disease, and a sustained state of inflammation is associated with a worse prognosis23. This may explain the higher SII values among HTN patients compared to healthy controls. It is also known that inflammation plays a role in decreasing kidney function24. In a population-based study, high SII values were independent predictors of increased urinary albumin excretion15. The results of the ENCORE trial showed that decreased renal function is associated with atherosclerotic cardiovascular disease, including increased CIMT values25. In a study conducted with HTN patients, a positive correlation was shown between the SII and CIMT26. These findings are similar to those of the present study.
In HTN, the level of inflammation increases with endothelial dysfunction caused by mechanical or shear stress, while the activation of immune cells increases due to the triggering of atherosclerosis27. The activation of inflammatory cells and the production of collagen and matrix proteins play important roles in atherosclerosis28,29. Since atherosclerosis is a chronic inflammatory process, platelet and neutrophil levels increase depending on the inflammatory mediators released. This activation can lead to exacerbation of inflammatory responses through the secretion of various mediators of inflammation30. Lymphocytes play a role in the regulation of cytotoxic and inflammatory responses, and lymphocyte count undergoes a significant decrease in the presence of inflammation31. When tissue damage occurs, macrophages accumulate in the damaged tissue due to the inflammatory response. During this process, interleukin-6 (IL-6) and tumor necrosis factor-α are secreted, and CRP synthesis is induced and increases with the progression of atherosclerosis32. These parameters have also been shown to play a role in hypertensive organ damage and they have been associated with increased inflammatory and apoptotic activation in patients with HTN4,33,34. These findings support the idea that the inflammatory response may be increased according to the presence or severity of AOD in cases of HTN. Moreover, there is a positive correlation between CRP and SII values. This finding is consistent with the increased threshold levels of the SII in predicting the presence and severity of AOD in the present study.
Based on the above-mentioned mechanisms, indices obtained from neutrophil, lymphocyte, and platelet levels may better reflect atherosclerotic processes and the presence of AOD. The previous studies have shown that PLR and NLR, which are indicators of inflammation, are associated with both endothelial dysfunction and AOD14,35. The SII may be a better prognostic indicator, because it includes all the components of both the NLR and PLR. A recent study investigated the relationship between the exaggerated morning BP surge, an important indicator of target organ damage and cardiovascular outcome, and SII values in patients with newly diagnosed HTN36. The SII values were shown to have better diagnostic performance than PLR and NLR in predicting exaggerated morning BP surges. In the present study, we found that SII values showed superior diagnostic performance compared to PLR and NLR. Our findings support the idea that the SII may be an important screening tool, particularly for the risk stratification of newly diagnosed patients with HTN, given the role of inflammation in HTN.
The most important limitation of this study is its retrospective design. Another limitation is that highly sensitive inflammatory markers, including cytokines, were not studied.
High SII levels were independent predictors of the presence and severity of AOD in patients with newly diagnosed treatment-naive HTN. Based on the prognostic role of inflammation in HTN, SII values showed a systemic increase with the severity of AOD relative to the number of organs involved and offered high diagnostic performance with significant thresholds for all grades of AOD.











 nueva página del texto (beta)
nueva página del texto (beta)