Introduction
Lactarius (Pers.) is a fungal genus belonging to the family Russulaceae with a wide global distribution, with 638 known species (Bánki et al., 2021). It is one of the most diverse and abundant groups of Basidiomycetes in temperate and alpine habitats (Borgen et al., 2006; Gardes & Dahlberg, 1996; Geml et al., 2009; Heilmann-Clausen et al., 1998; Knudsen & Vesterholt, 2008; Kühner, 1975; Laursen & Ammirati, 1982; Ohenoja & Ohenoja, 2010). These fungi play an important role as ectomycorrhizal symbionts, since they have been reported as late-stage colonizers of Pinaceae, Fagaceae, and Betulaceae in a wide range of ecosystems (Comandini et al., 2012; Rinaldi et al., 2008).
The species of the subsection Scrobiculati, which are members of the subgenus and section Piperites, are among the most noticeable and distinctive in temperate forests (Hesler & Smith, 1979). The subsection Scrobiculati comprises nine species and 10 varieties (Hesler & Smith, 1979), which have conspicuous yellowish-orange fruit bodies, white latex changing to yellow, and a scrobiculate stipe (Barge & Cripps, 2016; Hesler & Smith, 1979). Some species of this group have been taxonomically and systematically controversial because of their morphological variability. For example, a study of the subsection Scrobiculati in northwestern Europe (Scandinavia) recognized eight species: Lactarius scrobiculatus (Scop.: Fr) Fr, L. leonis Kytöv., L. olivinus Kytöv., L. tuomikoskii Kytöv., L. auriolla Kytöv., L. resimus (Fr.) Fr., L. aquizonatus Kytov., and L. citriolens Pouzar, five of which were proposed as new species (Kytövuori, 1984) that were added to the nine species and 10 varieties previously recognized by Hesler & Smith (1979). After a morpho-anatomical analysis, Kytövuori (1984) mentioned that L. scrobiculatus grows exclusively with Picea abies, and the descriptions of two varieties of this species (L. scrobiculatus 'var. scrobiculatus' sensu [Hesler & Smith, 1960] and L. scrobiculatus var. canadensis sensu [Hesler & Smith, 1979]) show higher similarity to European species (L. tuomikoskii and L. leonis, respectively) and do not correspond morphologically to L. scrobiculatus sensu stricto. As well, the author proposed that the species under this name in North America are non-existent, with L. alnicola (Hesler & Smith, 1960) being the closest species. Recently, Barge & Cripps (2016) studied at a morphological and molecular level the group of species close to L. scrobiculatus (L. alnicola, L. gossypinus, L. aff. olivinus, L. payettensis, L. scrobiculatus var. canadensis, L. scrobiculatus var. montanus, and L. aff. tuomikoskii) and found that they show conspecificity with European taxa such as L. auriolla, L. leonis, L. olivinus, and L. tuomikoskii (Barge & Cripps, 2016).
In Mexico, according to previous reports, there are four species (L. alnicola, L. mexicanus, L. resimus, and L. scrobiculatus) (Candusso et al., 1994; Kong, 1995; Montoya & Bandala, 1996) and two varieties (L. resimus var. regalis and L. scrobiculatus var. pubescens) (Montoya, 1992; Montoya & Bandala, 1996) of the subsection Scrobiculati. The conspicuous yellowish species with latex of the same color from the mountainous regions of central Mexico was originally reported as L. scrobiculatus (Singer, 1957). Lactarius mexicanus was reported as a new species in the subsection Scrobiculati, which apparently is exclusively associated with Abies religiosa and has macroscopic and microscopic characteristics that differentiate it from L. scrobiculatus, L. deterrimus, and L. intermedius (Kong & Estrada, 1994). In turn, L. alnicola is another species reported from Abies-Pinus forests (Montoya & Bandala, 1996) and Quercus forests (Candusso et al.,1994); however, the species reported are not consistent morphologically. Besides, no DNA sequences (from any region, including ITS) from type material collections have been reported thus far. A recent review of ectomycorrhizal fungi listed 10 species of Lactarius associated with oyamel trees (Abies religiosa) in Mexico, of which only L. mexicanus is mentioned in the subsection Scrobiculati (Oros-Ortega et al., 2017); nevertheless, there are no molecular studies supporting the molecular identity of these species or evidencing its symbiosis with Abies religiosa at the ectomycorrhizal level.
Molecular tools have been useful in establishing new species records in subsection Scrobiculati, Lactarius pseudoescrobiculatus, which is mostly distributed in the Mediterranean region of Europe and for which only morphological data were previously available (Polemis et al., 2019). We studied specimens of the genus Lactarius subsection Scrobiculati in Abies religiosa forests of the Cofre de Perote National Park (CPNP) with the purpose of contributing with new information on their ecology, distribution, and molecular data.
The objectives of the study were: (1) to determine the identity of the specimens of Lactarius subsection Scrobiculati by integrating morphological and molecular evidence, (2) to establish the phylogenetic position of the species and their affinity with related taxa, and (3) to update the information on the ecology and distribution of the species of Lactarius subsection Scrobiculati found in the Cofre de Perote National Park.
Materials and methods
Study area and survey
The study was conducted in the Abies religiosa forest located between 3150 m. a. s. l. and 3600 m. a. s. l. in the community of El Conejo within the Cofre de Perote National Park (CPNP), Veracruz, Mexico. The CPNP Management Program (Comisión Nacional de Áreas Naturales Protegidas [Conanp], 2015) stated that the study area contains four types of climates according to the Köppen classification system, modified by García (1981): semi-cold semi-humid with long fresh summers (Cb’(m)(f)), semi-cold temperate semi-humid with long fresh summers (Cb´(w2)), temperate humid (C(m)(f)), and temperate semi-humid (C(w2)) (Conanp, 2015). According to the database of the National Water Commission (Conanp) for the period 1991-2020 for the Perote region, the average temperature was 12.7 with average maximum temperatures of 21.7 and average minimum temperatures of 3.8 with average rainfall of 577.5 mm; the rock was extrusive igneous, and the soil type was andosol (Conanp, 2015).
We established three study sites (Site 1: 19° 32’ 02’’ N, 97° 08’ 50’’ W at 3150 m. a. s. l.; Site 2: 19° 31’ 18.8’’ N, 97° 09’ 29’’ W at 3200 m. a. s. l.; and Site 3: 19° 32’ 17.7’’ N, 97° 10’ 15.0’’ W at 3400 m. a. s. l.), selected based on previous studies (Andrade-Torres et al., 2015) where fruit bodies of different species of the genus Lactarius had been recorded. We carried out opportunistic surveys at each site every 15 days during the rainy season (August-November) from 2015 to 2018, gathering basidiomes near shoots and trees of Abies religiosa. We described the fresh basidiomes, then placed them in hermetic bags and/or wax paper, labelled with the collecting data, and stored them in a cool box at 4° C until observation in the laboratory.
The description of the macroscopic characters of the pileus, lamellae, context, and stipe of the basidiomes was carried out in INBIOTECA using a stereo microscope (NATIONAL DC3-420t, Texas, USA). Fragments of lamellae from each basidiome were mounted on microscope slides, and 50 spores were measured under a compound microscope (Iroscope Mod. MG-11TF, México). The basidiospores were exposed to Melzer’s reagent to record their coloration and ornamentation. We obtained the quotient Q by dividing the length and width of the measured spores, resulting in a mean value of Q (Q’) (Montoya et al., 2010). Basidia, basidioles, and cystidia were observed in Congo red, Methylene Blue, and 5% and 10% KOH, while laticiferous hyphae were observed in sulphovanillin. The terminology used in the description corresponded to that referred to by Heilmann-Clausen et al. (1998). After characterizing the basidiomes, they were dehydrated for preservation. To indicate the taxonomic status of the species studied, the MykoBank databases were revised (Robert et al., 2013). The collections formed part of the herbarium XAL of the Instituto de Ecología A. C., located in Xalapa, Veracruz, Mexico.
DNA extraction, PCR amplification, and sequencing
Total DNA extraction was performed by processing a fragment of the context (1 cm³) of the basidiomes from surveys RF96 and RF-116 with the Wizard Genomic DNA Purification Kit (Promega) according to the manufacturer’s protocol. The ITS region of the ribosomal DNA was amplified using the primers ITS1F and ITS4 (Gardes & Bruns, 1993; White et al., 1990). The final concentration of the PCR reaction was 1X MyTaq buffer (including Mg and dNTPs), 0.4 µM of each primer, 40 ng DNA, and 1 unit of MyTaq DNA polymerase (Bioline, USA Inc.). The PCR conditions follow the protocol by Gardes & Bruns (1993). The PCR products were observed in 1.5% agarose gel in 1X TAE with ethidium bromide. The PCR products were purified and sequenced using Sanger’s method at MACROGEN (Korea). The sequences were sent to GenBank® and deposited under the following accession numbers: Lactarius mexicanus: MF040818; and L. alnicola: MH820031.
Phylogenetic analysis
The consensus sequences of each basidiome were obtained by assembling the forward and reverse regions in the BioEdit sequence alignment editor version 7.1.9 (Hall, 1999). The generated ITS sequences were sent to BLASTn (Altschul et al., 1997) to find the sequences with high similarity. For the phylogenetic analysis, we selected from GenBank the sequences of species in the subsection Scrobiculati and in sister sections and subsections belonging to the subgenus Piperites, according to the classification system by Hesler & Smith (1979). The phylogenetic analysis consisted of 40 sequences of the rDNA-ITS region, with 666 characters in the final matrix representing 20 species of Lactarius, 19 of which corresponded to eight taxa located within the subsection Scrobiculati (Figure 1, bold arrow). Of these, 13 sequences comprising eight species had a European distribution (L. aquizonatus, L. auriolla, L. leonis, L. tuomikoskii, L. intermedius, L. pseudoescrobiculatus, and L. scrobiculatus) (Table 1, Figure 1). Five sequences corresponded to three species, one variety, and one related species with distribution in North America (L. aff. olivinus, Lactarius alnicola, Lactarius mexicanus, L. scrobiculatus). The other 19 sequences corresponded to seven species of the subgenus Piperites (L. yazooensis, L. zonarius, L. controversus, L. haugiae, L. psammicola, L. torminosus, L. acatlanensis, L. olivaceoumbrinus). The sequences of the endemic Mexican species Lactarius haugiae (GenBank: KT583640, KT583641, and KT583642) and L. acatlanensis (GenBank: KT583639, KT583645, KT583638, KT583643, KT583644, KT583646) were used in the phylogenetic analysis since they were among the few sequences of Lactarius in Mexico. Even though they did not belong to the subsection, they did belong to the subgenus and section Piperites. Two sequences of Lactarius crassus were used as an outgroup to root the tree, according to Polemis et al. (2019).
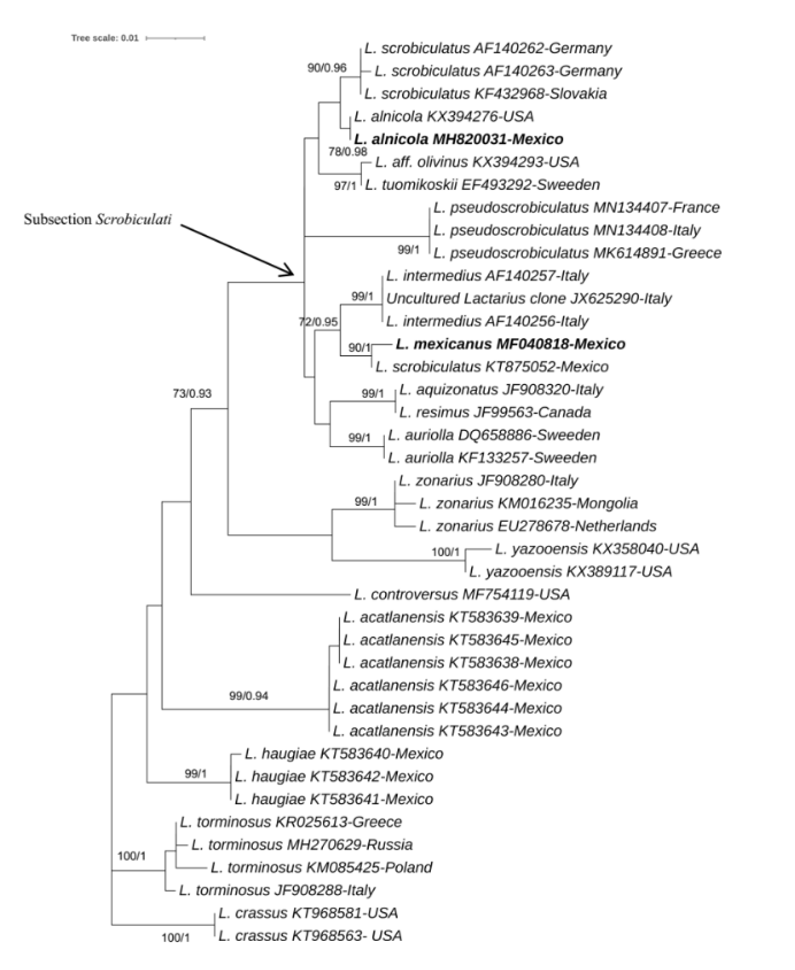
Source: Author’s own elaboration.
Figure 1 Phylogenetic relationships of Lactarius alnicola and L. mexicanus based on ITS sequences from basidiomes and inferred from the maximum likelihood tree, including bootstrap values (BS) (only values ≥70% are indicated) and Bayesian posterior probabilities (BPP) (only values ≥0.90 are indicated). Lactarius torminosus and L. crassus were used as outgroups. New sequences obtained here are indicated in bold. Specimen names, accession numbers of ITS and country of origin are indicated for each sequence.
Table 1 Information of the rDNA-ITS region sequences used in the phylogenetic analysis: taxa, voucher, locality, sequence accession number according to GenBank database, and associated phytobiont. The collections of the present study are indicated in bold.
| Taxa | Voucher | Country | Accession number | Associated phytobiont |
| L. acatlanensis | LM4657 | Mexico | KT583639 |
Fagus grandifolia var. mexicana, Quercus spp.,
Carpinus caroliniana |
| L. acatlanensis | LM5093 | Mexico | KT583645 |
Fagus grandifolia var. mexicana, Quercus spp.,
Carpinus caroliniana |
| L. acatlanensis | LM4655 | Mexico | KT583638 |
Fagus grandifolia var. mexicana, Quercus spp.,
Carpinus caroliniana |
| L. acatlanensis | LM5051 | Mexico | KT583643 |
Fagus grandifolia var. mexicana, Quercus spp.,
Carpinus caroliniana |
| L. acatlanensis | LM5053 | Mexico | KT583644 |
Fagus grandifolia var. mexicana, Quercus spp.,
Carpinus caroliniana |
| L. acatlanensis | VB4723 | Mexico | KT583646 |
Fagus grandifolia var. mexicana, Quercus spp.,
Carpinus caroliniana |
| L. aff. olivinus | EB0051-14 (MONT) | USA | KX394293 | Picea engelmannii |
| L. alnicola | EB0064-14 (MONT) | USA | KX394276 | Picea engelmannii |
| L. alnicola | RF-116 | Mexico | MH820031 | Abies religiosa |
| L. aquizonatus | 15167 | Italy | JF908320 | Not mentioned |
| L. auriolla | RW1601 (GENT) | Sweden | KF133257 | Not mentioned |
| L. auriolla | UP537 | Sweden | DQ658886 | Not mentioned |
| L. controversus | Mushroom Observer 248669 | USA | MF754119 | Soil under firs, pines, and poplars |
| L. crassus | Trappe 17996 | USA | KT968581 | Not mentioned |
| L. crassus | OSC 41826 | USA | KT968563 | Not mentioned |
| L. haugiae | LM4957 | Mexico | KT583640 | Quercus spp. and Carpinus caroliniana |
| L. haugiae | LM4988 | Mexico | KT583641 | Quercus spp. and Carpinus caroliniana |
| L. haugiae | LM4994 | Mexico | KT583642 | Quercus spp. and Carpinus caroliniana |
| L. intermedius | sporocarp | Italy | AF140256 | Abies alba |
| L. intermedius | mycorrhizae | Italy | AF140257 | Abies alba |
| L. mexicanus | RF-96 | Mexico | MF040818 | Abies religiosa |
| L. pseudoscrobiculatus | UP2014-12-02 | Italy | MN134408 | Not mentioned |
| L. pseudoscrobiculatus | G00126436//n SIB271003/2 | France | MN134407 | Not mentioned |
| L. pseudoscrobiculatus | EP.14-K302 | Greece | MK614891 | Not mentioned |
| L. resimus | DAVFP:26308 | Canada | JF899563 | Tsuga heterophylla |
| L. scrobiculatus | ue180 | Germany | AF140263 | Picea abies |
| L. scrobiculatus | fo46774 | Germany | AF140262 | Picea abies |
| L. scrobiculatus | HC-PNNT-045 | Mexico | KT875052 | Not mentioned |
| L. scrobiculatus | JN01-058 | Slovakia | KF432968 | Not mentioned |
| L. torminosus | No | Russia | MH270629 | Not mentioned |
| L. torminosus | 968 | Italy | JF908288 | Not mentioned |
| L. torminosus | JN11-086 | Greece | KR025613 | Not mentioned |
| L. torminosus | ID PAN 460 | Poland | KM085425 | Not mentioned |
| L. tuomikoskii | UP576 | Sweden | EF493292 | Not mentioned |
| L. yazooensis | 452 | USA | KX389117 | Not mentioned |
| L. yazooensis | MB684 | USA | KX358040 | Not mentioned |
| L. zonarius | 698 | Italy | JF908280 | Not mentioned |
| L. zonarius | 10162 | Mongolia | KM016235 | Birch forest |
| L. zonarius | UE27.09.2002-4 | Netherlands | EU278678 | Not mentioned |
| Uncultured Lactarius clone | M1MPB1 | Italy | JX625290 | Not mentioned |
Source: Author’s own elaboration.
The JModel ModelTest 2 (Darriba et al., 2012) was used to perform the best evolutionary model. The best fit model of nucleotide evolution using AIC (Akaike Information Criterion) was HKY+G (4400.35) for the dataset. The alignment was analyzed by Bayesian Inference (BI) and Maximum Likelihood (ML). BI analysis was used in Mr. Bayes version 3.2.7 for Windows (x64) (Ronquist et al., 2012) with settings as described by Montoya et al. (2010). ML was performed using MEGA X (Kumar et al., 2018) with 1000 bootstrap replicates following the ML heuristic method nearest neighbor interchange. Bootstrap values (BS) >75 and Bayesian posterior probabilities (BPP) > 0.90 were considered significant. The tree was visualized in MEGAX and edited in ITOL version 3 (Letunic & Bork, 2016).
Results
Macroscopic and microscopic characters
Description: The pileus is 53 mm-113 mm in diameter, subhemispherical to infundibuliform, centrally depressed, faintly zonate, viscid to glutinous; margin is straight to incurved, fibrillose tomentose, cuticle not easily removed, yellow (Figure 2 a-c). The lamellae are arched, thin, staining brownish where damaged, staining ocher-orange in 5% and 10% KOH (Figure 2 b-d), crowded; the latex is white, scarce, unchanging on exposure; the taste is astringent to strongly pungent reminiscent of ginger. The context is compact, rigid, whitish-yellowish. The stipe is 25 mm-60 mm x 17 mm-35 mm wide, yellow-orange to brown; the latex is white, unchanging on exposure to air. The stipe is cylindrical to curved, with light to ochraceous-yellowish scrobicules; the inner stipe is slightly to strongly fistulous, concolorous with the pileus. The rhizoids are small, erect, whitish-yellowish towards the base.
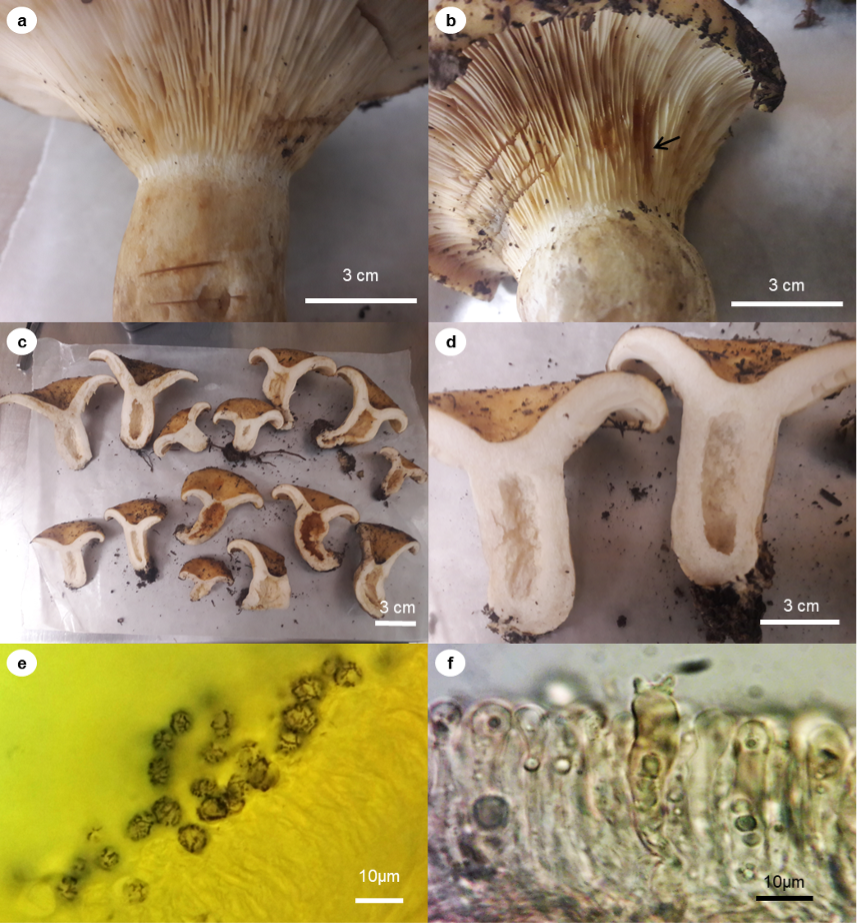
Source: Author’s own elaboration
Figure 2 Lactarius alnicola A.H. Sm. 1960. (a) Characteristics of the context and shape of the lamellae; (b) Reaction of the lamellae (arrow) to 5% KOH; (c) and (d) basidiomes; (e) spores 100X in Melzer’s; (f) basidium and pseudocystidia.
The spores are 7 µm-10 µm x 6 µm-7 µm (Q = 1.17-1.43, Q’ = 1.26), ellipsoid to broadly ellipsoid, hyaline, gutulated, amyloid ornamentation forming a partial reticulum with bands and isolated interconnections (Figure 2e). Basidia are 4-spored. Basidioles are clavate (Figure 2ff. Pleurocystidia is 55 µm-80 µm x 7 µm-10 µm, with apex attenuated to mucronate, scarce. No cheilocystidia or pseudocystidia are observed. Context is formed by irregular hyphae, with abundant laticifers 6 µm-9 µm wide, hyaline-yellowish but changing to brownish in Melzer’s reagent. The hymenium trama is irregular with hyaline-yellowish hyphae. The pellis forms a thick ixocutis of irregular, thin, hyaline-yellowish hyphae. Abundant laticifers in the hymenium trama like those in the context.
Its habit is ectomycorrhizal, associated with trees of Abies religiosa under the canopy at 3400 m. a. s. l. Soil type: andosol.
Examined material: MEXICO, Veracruz, Cofre de Perote National Park, community El Conejo, 22.XI.2015, RFGO 100 (XAL); loc. cit., 9.IX.2016, RFGO 116 (XAL).
This species is distributed in North America, principally in the United States under Picea engelmanii (Barge & Cripps 2016) and Alnus forests (Hesler & Smith, 1960; Hesler & Smith, 1979). In Mexico, L. alnicola was reported in Quercus spp. forests of Baja California (Ayala-Sánchez et al., 2015), in Pinus-Abies forests of Tlaxcala (Kong, 1995) and in Veracruz (Montoya & Bandala, 1996).
Description: the pileus is 59 mm-190 mm in diameter, subhemispherical, with a depressed center when young, becoming infundibuliform when mature (Figure a-f), azonate surface, with adpressed striations, moderately viscid; texture becomes slightly granulose after loss of moisture; the margin of pileus is slightly curled-in to recurved when mature, faintly fibrillose to glabrous, light yellow. The lamellae are adnate to subdecurrent, subdistant, with one to two lamellulae between the lamellae, bifurcate near the stipe, arched; the margin of the lamellae is smooth when young, becoming eroded when mature, with translucent structures projected outwards from the margin; the lamellae are cream white, yellowish to salmon. The context is white, with a change of coloration like the latex towards the margin, solid to hollow, odor fruity, more concentrated than in the pileus surface; the taste of the context is slightly astringent to fruity. The latex is white, changing to sulfur yellow on exposure to air and to orange in 10% KOH, with taste pleasant, fruity (Figure 3d). The stipe is 45 mm-80 mm x 18 mm-36 mm, cylindrical to subventricose, thick, slightly attenuated towards the base, straight to slightly curved, with irregular scrobicules towards the apex on the external side, concolorous with the pileus to pale grayish; the inner stipe is broadly fistulous to hollow when mature, with some salmon spots towards the base.

Source: Author’s own elaboration.
Figure 3 Lactarius mexicanus A. Kong & Estrada, Mycotaxon 52 (2): 446 (1994). (a-c) basidiomes in the field; (d) latex exuding from the lamellae (arrow); (e) context of the basidiome; (f) different developmental stages of the basidiomes; (g) amyloid spores in Melzer’s reagent; (h) hyaline yellowish ixocutis (ix) in 10% KOH.
Spores are 8 μm-10 μm x 7 μm-9 μm, subglobose to broadly ellipsoid (Q = 1.14-1.33, Q´ = 1.20), gutulated in 5% and 10% KOH, hyaline to hyaline-yellowish, amyloid ornamentation consisting of lines and ridges forming an open, partial, or completely interconnected reticulum or low ridges scattered or isolated in some areas of the spore (Figure 3g). The basidia are 4-spored, (45-) 47 μm-58 μm (-60) x 9 μm-12 μm, cylindrical to slightly clavate, with thin walls, hyaline-yellowish. The hymenium trama is composed of irregularly arranged hyphae. Pleurocystidia is 45 μm-56 μm (-75) x 9 μm-10 μm, scarce, nearly clavate, apex mucronate. Pilleipellis forms an ixocutis with thin, hyaline hyphae (Figure 3h); laticiferous hyphae are 2 μm-4 μm wide. Subpellis is irregular, plectenchymatous to parenchymatous.
Its habit is ectomycorrhizal, associated with trees of Abies religiosa under the canopy between 3150 m. a. s. l. and 3400 m. a. s. l. Soil type: andosol.
Material examined: MEXICO, Tlaxcala, Kong 2248 (TLXM; ISOTYPE: EIU, ENCB). Veracruz, Cofre de Perote National Park, community El Conejo, 27.IX.2005, RFGO 67 (XAL); loc. cit., 11.XI.2005, RFGO 78 (XAL); loc. cit., 15. VIII.2015, RFGO 96 (XAL); loc. cit., 25. VIII.2015, RFGO 99 (XAL); loc. cit., RFGO 103, 28.XI.2015 (XAL); loc. cit., RFGO 155, 30.X.2018 (XAL).
This species is distributed in Mexico City, Estado de Mexico, Hidalgo, Michoacán, Morelos, and Puebla, and it is associated with Oyamel fir (Abies religiosa) forests (Kong & Estrada, 1994).
Phylogenetic analysis
In the topology of the phylogenetic tree, the sequences of the taxa of the subsection Scrobiculati form a well-differentiated clade (Figure 1). The sequence generated from our studied specimen Lactarius alnicola (MH820031) robustly groups with the sequence of L. alnicola from Montana, USA (KX394276) (BS = 78, BPP = 0.98) (Figure 1). The sister clade is composed of European specimens of L. scrobiculatus (AF140263, AF140262, KF432968) from Germany and Slovakia (Figure 1).
The clade formed by Lactarius mexicanus (MF040818) and L. scrobiculatus (KT875052) shows robust values (BS = 90, BPP = 1) (Figure 1), and its sister group is composed of three sequences of L. intermedius (AF140256 and AF140257, obtained from basidiomes, and JX625290, obtained from the tip of a mycorrhized root; both basidiomes were collected in Abies alba forests in Italy) with well supported values (BS = 99, BPP = 1) (Figure 1).
Discussion
Based on the morphological and molecular evidence, the specimens of Lactarius subsection Scrobiculati collected in the Abies religiosa forest of the Cofre de Perote National Park correspond to Lactarius mexicanus and L. alnicola.
Lactarius mexicanus was proposed as a new Lactarius species from Mexico by Kong & Estrada (1994), finding it different from the North American L. scrobiculatus var. canadensis, L. scrobiculatus var. pubescens, and L. scrobiculatus var. scrobiculatus proposed by Hesler & Smith (1979), as well as from related European species (L. intermedius).
Our collected specimens identified as L. mexicanus are consistent with the macro- and microscopic characteristics that Kong & Estrada (1994) used to describe this species: large 70 mm-200 mm (-270 mm), with pale yellowish basidiomes, the latex that quickly changes to yellow, the pileus generally azonate, the lamellae are commonly bifurcate towards the base of the stipe, a long stipe 60 mm-100 mm (-180 mm), with scrobicules, subacrid to pleasant taste, relatively large spores (7.4 μm-) 7.9 μm-9.8 μm (-10.9) μm x (6.3 μm-) 6.6 μm-7.9 μm (-8.4 μm) (vs. 8 μm-10 μm x 7 μm-9 μm), and the macrocystidia (40 μm-) 50 μm-115 μm (-130 μm) x (6 μm-) 8 μm-13 μm (-17 μm), abundant pseudocystidia, and it is associated with A. religiosa; although our specimens exhibited shorter and scarcer cystidia (45 μm-50 μm x 9 μm-10 μm). It is not consistent with L. scrobiculatus var. pubescens since this variety has shorter stipes (15 mm-30 mm vs. 45 mm-80 mm), smaller spores (6 μm-7. 5 μm x 4.5 μm-6 μm vs. 8 μm-10 μm x 7 μm-9 μm), and it is associated with Pinus (Hesler & Smith, 1979) and differs from L. scrobiculatus var. canadensis, because this variety has “olive-buff” tones in the pileus, crowded lamellae, and a tomentose-fibrillose margin with appressed squamules (Hesler & Smith, 1979).
Our sequence of Lactarius mexicanus (GenBank: MF040818) has high values of ML bootstrap and BI probabilities (90/1 respectively) and similarity values of BLAST = 98.69% to the other sequences labeled as L. scrobiculatus (BoldSystem: ECMMX040-11). This sequence belongs to a basidiome reported from Nevado de Toluca Biosphere Reserve, Temascaltepec, Estado de México at 3222 m. a. s. l. However, there are no descriptions of this collected specimen. The phylogenetically closest group is composed of three sequences of L. intermedius (AF140256, AF140257, and JX625290) that were collected in Abies alba forests in Italy (Eberhardt et al., 2000) (Figure 1). This confirms what Kong & Estrada (1994) reported: that morphologically, and now demonstrated at a molecular level in the present study, L. intermedius is the European species closest to L. mexicanus. Records of L. mexicanus in Mexico have been reported in the Cofre de Perote (Andrade-Torres et al., 2015; Córdova-Chávez et al., 2014) and originally in La Malinche National Park (Kong & Estrada, 1994). In addition, according to the Biodiversity Information Facility (GBIF, 2024a), L. mexicanus has four recorded occurrences between the years of 1990 and 2024: the first and second occurrence in 1990 and 1995 in Abies-Pinus forests in La Malinche volcano in La Malinche National Park, the third in 2013 in the Nevado de Toluca National Park and the fourth in 2018.
Lactarius mexicanus has also been reported in Guatemala (Porras & Flores, 2016) in A. guatemalensis forests (Flores-Arzú, 2020). Based on these data, we can say that Lactarius mexicanus has a wide distribution in fir forests, but it is restricted altitudinally.
Lactarius alnicola is macroscopically characterized by a pale yellowish pileus, crowded lamellae, white latex unchanging on exposure or staining slightly yellowish, and an extremely pungent taste, and it is microscopically characterized by a thick ixocutis and spores with bands and warts that are mostly isolated rather than reticulate. Lactarius alnicola was reported by Hesler (1960) as a new species associated with Alnus sp. and was then divided into three varieties for North America by Hesler & Smith (1979).
There are two reports of this species in Mexico: Candusso et al. (1994) reported L. alnicola in oak forests; however, the characteristics of the latex, the reaction to KOH, and the globose shape of the spores do not agree with the original description by Hesler (1960) or with the one of the present study and, thus, it could be a different taxon, as discussed by Montoya & Bandala (1996). The other report was by Montoya and Bandala in 1996, who reported L. alnicola var. alnicola in conifer forests in the Cofre de Perote, which is like the specimen we describe macroscopically (color of the pileus, close lamellae, and white latex unchanging on exposure), microscopically (size and ornamentation of the spores), and ecologically (associated with Pinus-Abies forests).
The collections of Lactarius alnicola reported in the present study show higher macro- and microscopical similarities to that originally reported by Hesler (1960), Montoya & Bandala (1996), and Barge & Cripps (2016) in the size and color of the pileus, latex unchanging on exposure, and the size and ornamentation of the spores (Figure 2e).
Lactarius alnicola is mainly differentiated from L. scrobiculatus by a white latex unchanging on exposure and staining slightly yellow on white paper. The color change of the latex of the species surrounding Lactarius scrobiculatus reported by Kytövuori (1984) does not agree with our collections of L. alnicola. The character of color change of the latex appears to delimit or differentiate Lactarius alnicola from other taxa within the subsection Scrobiculati. According to the Global Biodiversity Information Facility (GBIF, 2024b), Lactarius alnicola has a wide distribution from the west of North America and Mexico under conifer trees, with 2907 georeferenced records between 1941-2024. L. alnicola occurs in humid areas, frequently along streams in spruce areas, possibly in the exclusive presence of Picea engelmannii; it fruits in summer and early autumn in the United States (Barge & Cripps 2016).
In Mexico, L. alnicola has been reported in Quercus spp. forests of Baja California (Ayala-Sánchez et al., 2015), in Pinus-Abies forests of Tlaxcala (Kong & Estrada, 1994), and in Pinus-Abies forests of the Cofre de Perote, Veracruz (Montoya & Bandala, 1996). These fungi have a preference for acid soils of andesitic type.
At a molecular and phylogenetical level, the sequence generated in the present study, identified as L. alnicola (MH820031) from basidiomes, is grouped in a clade together with the sequence of L. alnicola (KX394276), with values of ML bootstrap and BI probabilities of 78/0.98, respectively (Figure 1), and 100% similarity among them when comparing with BLAST tool. The analysis shows a sister clade composed of three sequences of L. scrobiculatus (AF140262, AF140263, and KF432968) (Figure 1). We did not find sequences of the type material of L. alnicola or its varieties in GenBank. Other works mention that L. alnicola forms a conespecific group with L. scrobiculatus sequences from Europe (Barge & Cripps, 2016). There are no sequences from Lactarius alnicola sensu Hesler and Smith type and its varieties. It is necessary to generate more DNA sequences from the type material to compare before making its final determination.
Lactarius alnicola and L. mexicanus are considered poisonous by the people of La Malinche National Park (LMNP) in Tlaxcala, who refer to them as “corneta venenosa” (poison bugle or venomous bugle) (Kong, 1995; Ramírez-Terrazo et al., 2021). Lactarius alnicola var. alnicola, reported from Cofre de Perote National Park (CPNP), has an unknown edibility status, likely due to its spicy and astringent taste (Montoya & Bandala, 1996). In the same region, L. mexicanus is regarded as an edible species known as “chivito” (little goat), while L. alnicola, known as “chivo loco” (crazy goat), is not edible due to its hard and fibrous texture (pers. comm.).
The taxonomic study of Lactarius alnicola and L. mexicanus holds great ecological importance, as both species form an obligate symbiotic interaction (ectomycorrhiza) with Abies religiosa, a tree species widely distributed in the Trans-Mexican Volcanic Belt (Farjon, 2013). However, the altitudinal range has been restricted by rising temperatures due to climate change, which could lead to the loss of the functional fungi associated with these forests.
Conclusions
The present study provides details of the phylogenetic position of L. mexicanus and L. alnicola, which expands the knowledge on the variation of these species, and it also contributes to the deposit in GenBank of the first sequences of basiodomes L. mexicanus and L. alnicola from Mexico. It is necessary to carry out studies on biogeographic and evolutionary aspects that will allow us to obtain a robust picture of the evolutionary histories and help generate robust inventories with morphological and molecular data of the involved symbionts.
Conflict of interest
All authors declare no conflict of interest.











 nueva página del texto (beta)
nueva página del texto (beta)


