+ The statement is endorsed by Colegio Mexicano de Cardiología Intervencionista y Terapia Endovascular (COMECITE).
Introduction
Percutaneous coronary intervention (PCI) is a worldwide non-surgical procedure that has become the prevalent revascularization method for the last forty years. It yields evident benefits, including efficacy, low complication rate, and minimal invasion; nonetheless, there is a justified research line to improve artery patency by reducing the rate of acute or delayed failure of the treated vessel.1
The current dominant strategy to improve PCI for long-lasting benefits is the drug-eluting stent (DES) due to significantly less target lesion revascularization (TLR),2 even though, the current in-stent restenosis (ISR) rate is ≈10%. Stents impose a foreign body, thrombosis risk or bleeding in high-risk patients, and jailing branches potential. The metal layer in the arterial wall becomes significant after second or more stents to treat (ISR).3 Another issue is small-vessel coronary artery disease (SVD) restenosis, which ranges from 25.8% with a stent to 34.2% in the balloon angioplasty population,4 and bifurcation lesion restenosis, ranging from 4.6-6.7% in the main branch and 13.2-14.7% in the side branch.5 Giuseppe Di Gioia et al. recently published a systematic review of clinical outcomes following bifurcation PCI techniques. They found a TLR of 10.2% with provisional stenting, 8.9% with the crush, 7.5% with culotte, 6% with double kissing (DK) crush, and 11.5% with T small protrusion (T/TAP).6
Drug coated-balloon (DCB) is a novel tool to reduce restenosis without leaving a foreign body and adding metal layers. It is supported by expert group consensus statements and updated report publications for almost a decade. The stent should be reserved exclusively for significant residual stenosis or flow-limiting dissection.7,8
The authors’ purpose is to review historical publications and meta analysis in every scenario to make agreements and recommendations.
Material and methods
The Mexican College of Interventional Cardiology and Endovascular Therapy (COMECITE for the name in Spanish: Colegio Mexicano de Cardiología Intervencionista y Terapia Endovascular) formed the consensus group with a designated chairman and co-chairman who later distributed functions to the rest of the members. Every member searched and analyzed relevant publications about coronary DCB in ISR, SVD, and bifurcation settings, using Cochrane Handbook9 for systematic reviews of interventions and AMSTAR 2 (a measurement tool to assess systematic reviews) which is a critical appraisal tool for systematic reviews that includes randomized or non-randomized study trials of healthcare interventions.10 The members also reviewed single papers regarding special DCBs for special anatomical conditions. The consensus group discussed every paper in an expert panel format, nominal group technique, and anonymous Dolphy survey.11
The authorship for publication follows the International Committee of Medical Journal Editors (ICMJE).12
Statistical analysis
The consensus group used the Forest Plot13 with Number Cruncher Statistical Systems (NCSS) program and the Funnel Plot14 with the NHS improvement program. The consensus considered TLR as an effective endpoint and major cardiovascular events (all-cause or cardiovascular death, myocardial infarction, or target lesion thrombosis) as a safety endpoint. DCB vs DES trials analyzed ISR, SMD, and bifurcation lesion scenarios.
Tables 1 to 3 shows the analysis with the Cochrane Manual of systematic reviews of interventions and AMSTAR 2. Funnel plots and the Egger test15 explored publication bias or small-study effect. The Funnel plot is a graphic design that verifies deviations in the publications in systematic reviews and meta-analyses. High-precision trials are in the triangle’s midline, while low precision is in the triangle’s lateral regions.
Table 1: In-stent restenosis trials.
| Trial | Design | Year | n | Scenario | Material |
|---|---|---|---|---|---|
| AGENT(43) | 1:1, Open-Label, Core Lab, CEC | 2019 | 125 | Restenosis in-stent BMS or DES | PCB: 3 µg/mm2 iopromide, ATBC 2 µg/mm2 |
| BIOLUX-RCT(44) | 2:1, Open-Label, Core Lab, CEC | 2018 | 229 | Restenosis in-stent BMS or DES | SES, PCB 3 µg/mm2 BTHC |
| DARE(45) | 1:1, Open-Label, Core Lab, CEC | 2018 | 278 | Restenosis in-stent BMS or DES | EES, PCB 3 µg/mm2 iopromide |
| ESSENTIAL(46) | Open-Label, Core Lab, CEC | 2019 | 30 | Restenosis in-stent BMS or DES | 3 µg/mm2 paclitaxel organic ester |
| ISAR-DESIRE(47) | 1:1, Open-Label, Core Lab, CEC | 2013 | 340 | Restenosis in-stent DES | PES, PCB 3 µg/mm2 iopromide |
| PACCOCATH(48) | 1:1, Open-Label, Core Lab, CEC | 2006 | 52 | Restenosis in-stent BMS and DES | PCB 3 µg/mm2 iopromide and POBA |
| PEPCAD II(49) | 1:1, Open-Label, Core Lab, CEC | 2009 | 131 | Restenosis in-stent BMS | PES, PCB 3 µg/mm2 iopromide |
| PEPCAD CHINA(50) | 1:1, Open-Label, Core Lab, CEC | 2014 | 221 | Restenosis in-stent DES | PES, PCB 3 µg/mm2 iopromide |
| RESTORE(51) | 1:1, Open-Label, Core Lab, CEC | 2018 | 172 | Restenosis in-stent DES | EES, PCB 3 µg/mm2 iopromide |
| RIBS IV(52) | 1:1, Open-Label, Core Lab, CEC | 2015 | 309 | Restenosis in-stent DES | EES, PCB 3 µg/mm2 iopromide |
| RIBS V(53) | 1:1, Open-Label, Core Lab, CEC | 2014 | 189 | Restenosis in-stent BMS | EES, PCB 3 µg/mm2 iopromide |
| SEDUCE(54) | 1:1, Open-Label, Core Lab, CEC | 2014 | 50 | Restenosis in-stent BMS | EES, PCB 3 µg/mm2 iopromide |
| TIS(55) | 1:1, Open-Label, Core Lab, CEC | 2016 | 136 | Restenosis in-stent BMS | EES, PCB 3 µg/mm2 iopromide |
CEC = clinical events committee. BTHC = butyryl-tri-hexyl-citrate. ATBC = acetyl tributyl citrate. BMS = bare metal stent. DES = drug-eluting stent. PCB = paclitaxel-coated balloon. SES = sirolimus-eluting stent. EES = everolimus-eluting stent.
Table 2: Small vessel disease trials.
| Trial | Design | Year | n | Scenario | Material |
|---|---|---|---|---|---|
| BASKET 2(56) | 1:1, Open-Label, Core Lab, CEC | 2018 | 758 | Small vessel | EES, PES, PCB 3 µg/mm2 iopromide |
| BELLO(57) | 1:1, Open-Label, Core Lab, CEC | 2012 | 182 | Small vessel | BMS, PES |
| PEPCAD(58) | Open-Label, Core Lab, CEC | 2010 | 118 | Small vessel | PCB 3 µg/mm2 iopromide, BMS bailout |
| PICCOLETO(59) | 1:1, Open-Label, CEC | 2010 | 57 | Small vessel | PES, PCB 3 µg/mm2 PTX DMSO (d(i)m(ethyl)s(ulf)o(xide) |
| PICCOLETO II(60) | 1:1, Open-Label, CEC | 2020 | 214 | Small vessel | EES, PCB ≈2 µg/mm2 |
| RESTORE(61) | 1:1, Open-Label, Core Lab, CEC | 2018 | 230 | Small vessel | EES, PCB 3 µg/mm2 iopromide |
CEC = clinical events committee. EES = everolimus-eluting stent. PES = paclitaxel-eluting stent. PCB = paclitaxel-coated balloon. BMS = bare metal stent. PTX DMSO = paclitaxel dimethyl sulfoxide.
Table 3: Bifurcation trials.
| Trial | Design | Year | n | Scenario | Material |
|---|---|---|---|---|---|
| PEPCAD V(62) | Open-Label, Core Lab, CEC | 2011 | 28 | DCB in MB and SB + BMS in MB | BMS, PCB: 3 µg/mm2 iopromide |
| DEBUIT(63) | 1:1:1, Open-Label, Core Lab, CEC | 2012 | 117 | 1. DCB in MB and SB + BMS in MB 2. BMS in MB and POBA in SB 3. PES in MB and POBA in SB |
PES, BMS, PCB 3 µg/mm2 PTX DMSO |
| BABILON(64) | 1:1, Open-Label, Core Lab, CEC | 2014 | 108 | 1. DCB in MB and SB and BMS in MB 2. Provisional T stent with DES |
DES, BMS, PCB 3 µg/mm2 iopromide |
| DEBSIDE(65) | Open-Label, Core Lab, CEC | 2015 | 50 | PES in MB and DCB in SB | PES, PCB 2.5 µg/mm2 BTHC |
| SARPEDON(66) | Open-Label, CEC | 2015 | 50 | DES in MB and DCB in SB | DES, PCB 3 µg/mm2 BTHC |
| PEPCAD-BIF(67) | 1:1, Open-Label, Core Lab, CEC | 2016 | 64 | DES in MB and 1. POBA in SB 2. DCB in SB |
PCB 3 µg/mm2 iopromide |
CEC = clinical events committee. PCB = paclitaxel-coated balloon. MB = main branch. SB = side branch. BMS = bare metal stent. POBA = plain old balloon angioplasty. DES = drug-eluting stent. PES = paclitaxel-eluting stent. BTHC = butyryl-tri-hexyl-citrate. DCB = drug-coated balloon. PTX DMSO = paclitaxel dimethyl sulfoxide.
The Forest Plot, known as the Blobbogram, is a graph showing the results of a group of trials evaluating three aspects: the intervention’s neutrality, superiority, or inferiority through the odds ratio (OR). On the other hand, the heterogeneity index analyzes the clinical and methodological differences between the participants and interventions. The criterion is χ2 < 0.05 or inconsistency index, whose formula is I2 = (Q-df/Q) x100%. Heterogeneity is low at approximately 25%, moderate at approximately 50%, and high at approximately 75%.
Results
The consensus analyzed thirteen trials with 2,262 patients for ISR, six trials with 1,559 patients for SMD, six trials with 417 patients for bifurcation lesions, one meta-analysis comprising 5,711 patients with diverse bifurcation techniques, and several single papers for particular scenarios.
In-stent restenosis
The comparative trials’ efficacy endpoint for ISR indicates no significant difference between DCB versus DES 1.26 OR (95% confidence interval, 0.80-1.99, p = 0.07) with a 47.89% heterogeneity index (moderate) (Figure 1). The comparative trials’ safety endpoint for ISR indicates no significant differences between DCB versus DES 0.79 OR (95% confidence interval, 0.55-1.15, p = 0.22) with a 0% heterogeneity index (Figure 2). The funnel plot efficacy showed a low precision in the ISAR-DESIRE 3 DCB trial (Figure 3), and the TIS DES trial for safety (Figure 4).
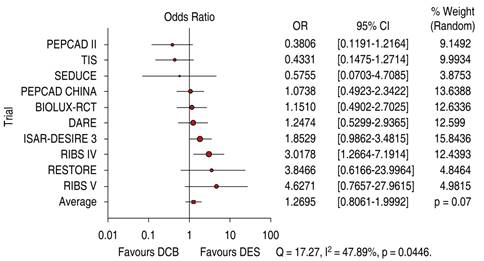
Figure 1: Target lesion revascularization for in-stent restenosis; DCB versus DES. DCB = drug-coated balloon. DES = drug-eluting stent.

Figure 2: Major cardiovascular events for in-stent restenosis; DCB versus DES. DCB = drug-coated balloon. DES = drug-eluting stent.
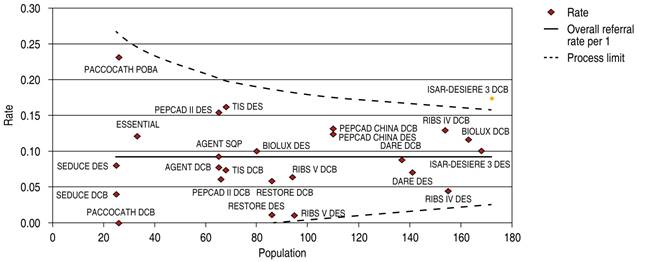
Figure 3: Funnel plot, TLR in ISR; DCB versus DES. TLR = target lesion revascularization. ISR = in-stent restenosis. DCB = drug-coated balloon. DES = drug-eluting stent.
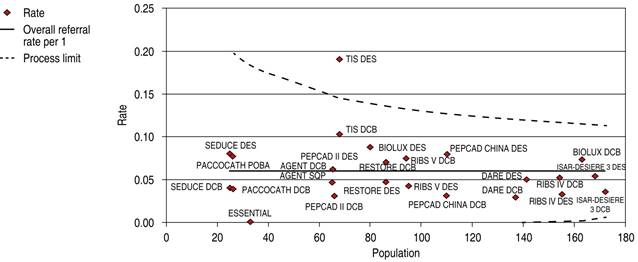
Figure 4: Funnel plot, MACE in ISR; DCB versus DES. MACE = major adverse cardiovascular events. ISR = in-stent restenosis. DCB = drug-coated balloon. DES = drug-eluting stent.
The DEB-Dragon-Registry, comprising 1,117 patients showed similar TLR (11.2% versus 11.2%; HR, 0.91 [95% CI, 0.55-1.51], p = 0.707), target vessel revascularization (TVR) (13.4% versus 14.2%; HR, 0.86 [95% CI, 0.55-1.36], p = 0.523), and device-oriented composite endpoint (14.2% versus 14.2%; HR, 0.91 [95% CI, 0.58-1.42], p = 0.667) between the thin-strut DES and DCB, respectively after propensity score matching. The device-oriented composite endpoint comprised cardiac death, target lesion revascularization, and target vessel myocardial infarction, together.16
Small vessel disease
The comparative trials’ efficacy endpoint for SVD indicates no significant differences between DCB versus DES 1.05 OR (95% confidence interval, 0.60-1.86, p = 0.97) with a 24.38% heterogeneity index (low) (Figure 5). The comparative trials’ safety endpoint for SVD indicates no significant differences between DCB versus DES 0.95 OR (95% confidence interval, 0.56-1.61, p = 0.76) with a 20.91% heterogeneity index (low) (Figure 6). The Funnel Plot for efficacy, showed low precision in PICCOLETO DCB, PEPCAD DCB + BMS, BASKET 2 DES, and BASKET 2 DCB trials (Figure 7), and in PICCOLETO DCB, RESTORE DCB, and RESTORE DES trials for safety (Figure 8).
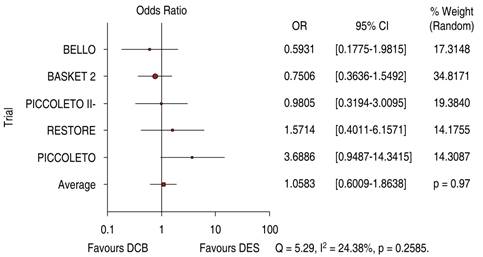
Figure 5: Target lesion revascularization for SVD; DCB versus DES. SVD = small vessel disease. DCB = drug-coated balloon. DES = drug-eluting stent.
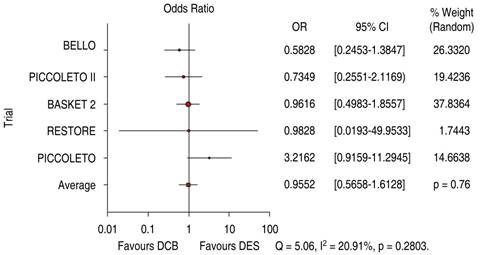
Figure 6: Major cardiovascular events for SVD; DCB versus DES. SVD = small vessel disease. DCB = drug-coated balloon. DES = drug-eluting stent.
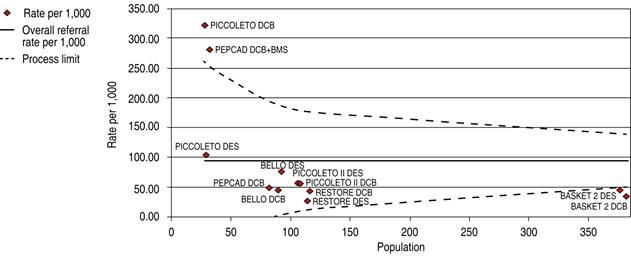
Figure 7: Funnel plot, TLR in SVD; DCB and DES. TLR = target lesion revascularization. SVD = small vessel disease. DCB = drug-coated balloon. DES = drug-eluting stent. BMS = bare metal stent.
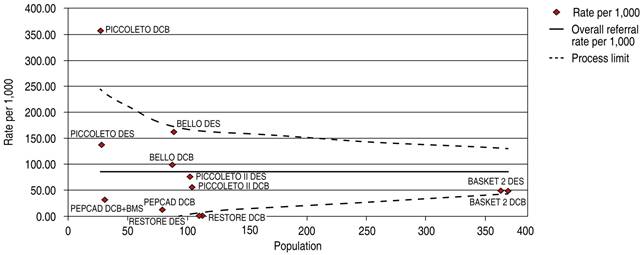
Figure 8: Funnel plot MACE in SVD; DCB and DES. MACE = major adverse cardiovascular events. SVD = small vessel disease. DCB = drug-coated balloon. DES = drug-eluting stent. BMS= bare metal stent
Her et al, in retrospect, enrolled 227 patients according to reference vessel diameter (RVD) > 2.5 and < 2.5 mm. The primary endpoint was late lumen loss after six months of follow-up. The secondary endpoint was target vessel failure where no differences were observed.17
Lesions in bifurcations
DES is significantly superior to DCB in bifurcation technique efficacy endpoint 1.56 OR (95% confidence interval, 0.59-4.10, p = 0.006) with heterogeneity index (high) (Figure 9). DES is significantly superior to DCB in bifurcation technique safety endpoint 1.49 OR (95% confidence interval, 0.91-2.42, p = 0.02) with a 60.63% heterogeneity index (moderate) (Figure 10). The Funnel Plot found low precision in the DEBUIT BMS + POBA trial for efficacy (Figure 11) and the DEBUIT BMS + POBA trial for safety (Figure 12).
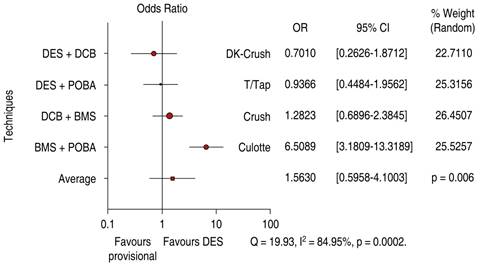
Figure 9: Target lesion revascularization for bifurcation lesions; DCB versus DES. DCB = drug-coated balloon. DES = drug-eluting stent.
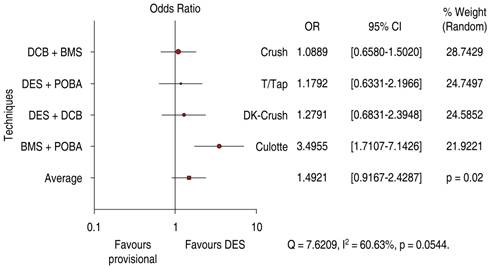
Figure 10: Major cardiovascular events for bifurcation lesions; DCB versus DES. DCB = drug-coated balloon. DES = drug-eluting stent.
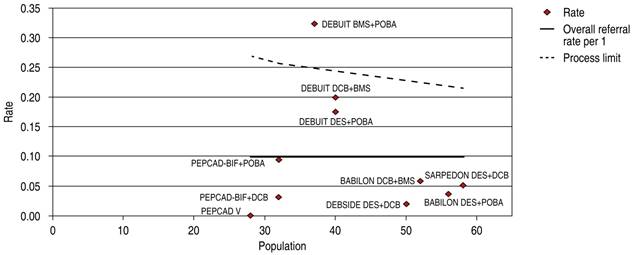
Figure 11: Funnel plot TLR in bifurcations; DCB and DES. TLR = target lesion revascularization. DCB = drug-coated balloon. DES = drug-eluting stent.
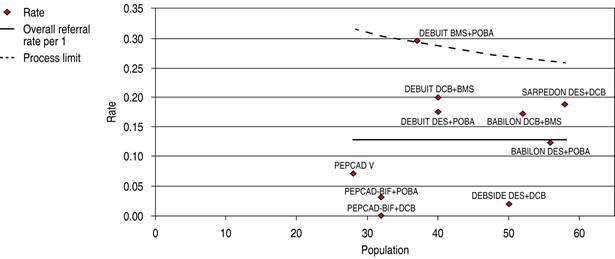
Figure 12: Funnel plot MACE in bifurcations; DCB and DES. MACE = major adverse cardiovascular events. DCB = drug-coated balloon. DES = drug-eluting stent.
BMS + POBA vs Culotte technique shifts efficacy and safety favor the 2 DES technique, but DCB provisional stenting has no significant differences from DK-Crush and Crush techniques.
Several metanalyses report significantly less lumen loss of DCB versus plain old balloon dilatation in the side branch and safe outcomes when treating the main vessel.18-21 DCB appears to be a good option for the «keep it simple and safe» principle of the European Bifurcation Club.22
ISR in left anterior descendent coronary artery
Prado Jr et al, in 2014, published a case report of ISR in ostial left anterior descendent (LAD), using a paclitaxel-eluting balloon, after seven months of follow-up the patient was symptom-free and had no angiographic restenosis.23 Yee ST et al, in 2021, presented an ostial LAD ISR treated using a DCB, with success.24
ISR in the left main coronary artery
Shetty R et al. published a case report of ISR in an unprotected left main bifurcation and previously failed venous grafting two years before, using sirolimus-eluting kissing balloons. The patient remained asymptomatic with a negative stress test after a six-month follow-up.25
Maximkin et al. reported a 4-year follow-up of DCB in the treatment of left main coronary artery bifurcation. Provisional T stenting is associated with a significantly lower frequency of MACE and side branch restenosis compared with the two-stent technique.26 Kitani et al. evaluated the efficacy and safety of DCB after directional coronary atherectomy (DCA) with a 12-month follow-up in 129 patients. They concluded that DCA/DCB provides good clinical outcomes and minimal side branch damage.27
Acute myocardial infarction
Several publications inform comparable results in AMI, for DCB versus DES in single centers for AMI.28
The REVELATION trial compared fractional flow reserve (FFR) after randomized treatment with plain balloon angioplasty on 120 patients who received either DCB or DES during ST-segment elevation AMI intervention. Thrombus aspiration was performed in 78% with DCB and 83% with the DES group; 18% of suboptimal results received a bare-metal stent, and after nine months of follow-up, the DCB strategy was non-inferior to DES, and seemed to be safe and feasible.29 This justifies the more recent finding of better one-year lumen loss for DCB versus DES in the setting of ST-elevation AMI (-0.12 ± 0.46 mm versus 0.14 ± 0.37 mm, p < 0.05), without significant major adverse events (11% versus 12%).30
The PEPCAD NSTEMI trial compares DCB versus BMS and DES in Non-ST-elevation AMI in 210 patients of whom, 62% have a multi-vessel disease and 31% were diabetics. 104 were randomized to DCB and 106 to stent treatment, 56% were treated with BMS, and 44% DES. After nine months, the primary endpoint was target lesion failure (cardiac or unknown death, reinfarction, and target lesion revascularization). Secondary end-points included major adverse cardiovascular events. DCB was non-inferior to stenting with BMS or DES.31
Discussion
DCB treatment requires an initial dilatation with semi, non-compliant, or super high-pressure balloon on a 1:1 balloon-vessel ratio. Residual stenosis ≤ 30%, absence of flow-limiting dissection, and guide-wire evaluation with FFR ≥ 0.75 or iFR ≥ 0.89 would be considered a good result for DCB deployment preparation. Intracoronary image improves plaque evaluation and treatment with cutting or scoring balloon, ablation, or shockwave; DCB inflation duration must last at least sixty seconds at nominal pressure.32,33 FFR predicts clinical outcome after balloon angioplasty with event-free survival rates at 6, 12, and 24 months of 92 ± 5%, 92 ± 5%, and 88 ± 6%, respectively, for FFR ≥ 0.90 after plain old balloon coronary angioplasty.34
There is significant coronary stenting contraindication or stent-less preference for several reasons such as planned non-cardiac surgery, high bleeding, and lesions that easily develop stent fracture. Iijima et al. published their experience in 118 de novo lesions not suitable for DES implantation 40% with very small vessel disease, 3% planned non-cardiac surgery, and 19% for high bleeding risk. TLR was the primary endpoint and suboptimal lesion preparation before DCB treatment was the secondary endpoint. Optimal lesion preparation is defined as TIMI flow grade 3, minor coronary dissection and residual stenosis ≤ 30%, suboptimal lesion preparation was 2.5%, three patient bailout stenting, 115 patients treated with DCB. TLR occurred in eight patients after an 8-month follow-up. Intracoronary image, Rotablator, conventional, cutting, and non-slip element balloon were used to optimize lesion preparation; they concluded DCB should be considered initially.35 Ybarra et al case report suggested DCB benefits from plaque modification strategy during staged CTO treatment.36
Dissection after plain balloon angioplasty is a problem that may cause discomfort and overwhelm in some operators who use unnecessary coronary stenting, especially after good prognostic mild A and B-type dissections. DEBATE II showed that moderate dissections (type C) had a better prognosis if restrained for further treatment, including stenting; the explanation may relate to possible positive remodeling and major late luminal gain.37,38
DCB and DES in TLR effectiveness and safety are similar. RIBS IV trial is the only one with a statistically significant difference; however, its development did not follow the German consensus recommendations emphasizing the 1:1 balloon-to-vessel ratio; on the other hand, the funnel plot heterogeneity analyses index is moderate in efficacy with 0% heterogeneity in safety.
DCB and DES in SVD efficacy and safety are comparable without statistically significant differences and a wide heterogeneity index. The PEPCAD trial utilized BMS as a bailout with very high restenosis (45%); in the PICOLLETO 2 trial, DCB was superior to the everolimus-eluting stent considering in-lesion late luminal loss and comparable in clinical outcomes.
The BASKET-SMALL 2 Trial showed similar outcomes on SVD treated either with DCB or DES, without significant difference in diabetic versus non-diabetic patients. However, the people with diabetes had less target vessel revascularization with DCB.39
Bifurcation trials significantly differ in effectiveness and safety between DCB and DES, showing high and moderate heterogeneity indices respectively. DES is superior to BMS and plain old balloon angioplasty (POBA), and DK-Crush has the lowest TLR in DES techniques, highlighted as the gold standard DES procedure, even though compared with DCB there is no significant difference.
The DEFINITION-II Trial informs significant improvement in clinical outcomes by comparing the double stenting technique vs provisional stenting in complex coronary bifurcation lesions. In this trial, 22.5% of the provisional stent group required a side branch stent,40 limiting the reliability of the final results.
The difference between regular balloon primary angioplasty versus stenting in ST-elevation myocardial infarction involves further interventions but there are no significant differences in mortality or reinfarction.41 Coronary stenting during ST-elevated AMI has several inconveniences, especially related to insufficient time to know every detail of the patients and how many times a bleeding tendency is evident after stent placement. On the other hand, the generalized vasoconstriction precludes the accurate reference diameter, causing the risk for undersized stents. These issues might be the reason for more major adverse events and cardiac death for DES treatment to ST-elevation AMI.42
The main findings of this consensus are:
DCB and DES are equally effective and safe in IST. The clinical advantages of DCB include less risk for stent thrombosis, less bleeding in high-risk patients, shorter time of dual or triple antiplatelet therapy, avoidance of metal layers, and jailing side branches.
DCB in vessels with a diameter less than 3 mm (SVD) is a good option; the PICOLLETO-2 trial reports significantly less lumen loss with DCB vs everolimus-eluting stent and comparable clinical outcomes.
DCB in bifurcations is a promising tool. Provisional stenting in non-complex bifurcation lesions showed long-term benefits compared to a two-stent strategy.
Recommendations
The target lesion must receive proper preparation before DCB deployment: pre-dilatation with the conventional balloon, semi-compliant, non-compliant, or super high pressure could be used with a balloon-to-vessel ratio of 1:1, ≤ 30% residual stenosis, and dissection type A, B, C, and E is permitted. Consider prolonged inflation at low pressure for severe dissections before stenting.
The preparation may include plaque ablation with a cutting balloon, scoring balloon, laser, and shockwave.
The DCB must receive gentle management, avoiding touching the balloon and avoiding any friction with the system.
Consider DCB as a stand-alone therapy in de novo lesions in segment or bifurcation lesions for high-risk bleeding patients.
DCB should be the standard method to treat restenosis from either BMS or DES, equivalent to DES but nothing left behind.
Consider DCB over DES to treat de novo lesions in small vessels, especially in people with diabetes and bifurcations.
Consider DCB in potential stenting complications such as severe angulation, angle difference, bifurcation with > 50% side branch stenosis, hinge motion, severe calcification, chronic total occlusion (CTO), eccentricity, and atherosclerotic lesion associated with myocardial bridging.
Consider DCB for multi-vessel coronary artery disease.
Consider DCB in acute coronary syndromes, including ST and non-ST elevation myocardial infarction and thrombus aspiration needed before DCB deployment.
Consider pressure wire, a physiological assessment for better outcomes. FFR ≥ 0.84 or iFR ≥ 0.89.
IVUS or OCT aid to treatment evaluation is not essential after DCB deployment unless suboptimal results or complications and pre/post stenting.
Consider being part of a DCB registry.











 nueva página del texto (beta)
nueva página del texto (beta)


