Introduction
Amyloidosis corresponds to a group of diseases in which there is an extracellular tissue deposition of fibrillar proteins called amyloids, which originated from an alteration in the folding of specific protein precursors. Amyloidosis ends up infiltrating and damaging the function of various organs such as the kidneys, liver, nervous system, and heart. When it invades the myocardium, it can compromise ventricular function (initially with diastolic dysfunction) and the conduction system, leading to a poor prognosis for the patient.1
In a non-systematic search of the literature using the terms «amyloidosis», «cardiac» and «compromise» in PubMed with the Mesh Terms: Heart and Amyloidosis, multiple reports of cardiac amyloidosis in American and European populations were found, but there were no case reports of cardiac amyloidosis, as an acute presentation of heart failure, in elderly patients in Latin America. However, here is a case of an elderly patient who debuted with de novo acute heart failure. Amyloid deposits were documented without other etiologies that explained her acute decompensation.
Case presentation
A 76-year-old female patient with a history of arterial hypertension and difficult-to-manage atrial fibrillation (AF), anticoagulated with rivaroxaban, was admitted to the institution for 1-month for moderate dyspnea (mMRC 4) with decreased functional class, paroxysmal nocturnal dyspnea, orthopnea, bendopnea, lower limb edema grade III. With no history of ischemic heart disease, on physical examination with normal blood pressure and preserved ventricular function.
The patient first received symptomatic treatment with diuretics for dyspnea. An electrocardiogram (EKG) (Figure 1) and transthoracic echocardiogram (Figure 2) were performed for diagnosis.
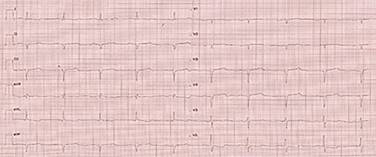
Figure 1: Electrocardiogram with the low-voltage QRS complex. Q waves in precordial leads simulating an old anteroseptal acute myocardial infarction (AMI).

Figure 2: Transthoracic echocardiogram. Apical four-chamber view showing left ventricular hypertrophy and left atrial enlargement.
The EKG showed Q waves in precordial leads from V1 to V4 with associated low voltage. The echocardiography showed a left ventricular ejection fraction (LVEF) of 48%, grade II diastolic dysfunction, biauricular dilatation, and ventricular hypertrophy with a septal predominance (thickness of 16 mm). Those characteristics are related to restrictive heart disease. In addition, an increase in cardiac tissue refringence was evidenced; there was not a specific strain pattern reported by the echocardiography. Other laboratory tests were normal, such as hemoleukogram, electrolytes, renal function, thyroid function, and glycemia.
Given these clinical and paraclinical findings, the Cardiology and Hemodynamics services decided to perform coronary arteriography to rule out coronary ischemic disease. Coronary arteries without significant obstructive lesions were reported. With this new information and associated with the EKG and echocardiography findings, an endomyocardial tissue biopsy was performed. The results showed positivity for red congo and apple-green birefringence with polarizing light, confirming the involvement of amyloid deposition. In an effort to define the extent of cardiac involvement, cardiac magnetic resonance imaging (MRI) was requested, but unfortunately, the patient’s clinical condition worsened, and she died after the diagnosis was established.
Discussion
Amyloidosis with cardiac involvement is divided into two main types that account for 95% of all cases. Amyloid light-chain or primary amyloidosis (AL amyloidosis) is explained by a clonal alteration of plasma cells due to the overproduction of light chains. On the other hand, Transthyretin Amyloidosis (ATTR) is produced by the misfolding of the hepatic protein transthyretin and can be acquired senile (ATTRwt) or hereditary (hATTR).2 The prevalence of this pathology is relatively low, or it is underdiagnosed since deposits of the senile form have been found in up to 25% of older patients, most of whom are asymptomatic.3 This prevalence has been increasing over the years due to the development of better diagnostic methods, greater knowledge of the disease, and a bigger number of patients (greater aging of the population).
Clinical diagnosis is not easy because of the wide spectrum of clinical presentations. Amyloidosis is usually presented as heart failure with preserved ejection fraction, dyspnea associated with exercise, AF, left branch bundle block, cerebrovascular events, or symptoms of right heart failure such as lower limb edema and ascites.4 Paraclinical tests are fundamental; EKG shows lower voltage QRS complex or pseudo-infarction patterns, as reported, to confirm the diagnosis. AF may also be evidenced, which is relatively common, with a prevalence of 10 to 20% and significant morbimortality.5
The cornerstone in the diagnosis of amyloidosis is echocardiography. It provides characteristic patterns: concentric growth of the left ventricle with a more echogenic appearance than in patients with true hypertrophy. This is generated because amyloid deposits produce enlargement of the interventricular septum of more than 12 mm and give a granular appearance to the myocardium.6 As for the atria, a restrictive component can be appreciated with enlargement of chambers, contractility dysfunction in the absence of the transmitral A wave, and the tissue Doppler A wave in patients with sinus rhythm, in addition to an increased ventricular E/e ratio indicating an elevated ventricular pressure.7 It should be emphasized that the Doppler findings depend on the patient’s stage, demonstrating progressive infiltration in accordance with diastolic dysfunction.8 The regional Strain is also an invaluable help since it has characteristics only seen in a few pathologies. The amyloidosis case is presented as a global compromise that preserves the apical zone in the bull’s eye or Japanese flag.9
In the case described, several elements led to suspicion of cardiac amyloidosis, such as low-voltage QRS complexes, ventricular thickening with septal predominance, and increased echogenicity of the left ventricle. Unlike hypertrophic cardiomyopathy, which generates high voltages, amyloidosis infiltration generates low voltage.
Other imaging methods can be very useful, such as cardiac MRI, which shows diffuse late gadolinium enhancement in the subendocardium that does not follow the coronary distribution and has a sensitivity of 93%. Another imaging- resource is cardiac scintigraphy with Tc99m. It is a less invasive study compared to endomyocardial biopsy, which requires the injection of a radiotracer. Once injected, it binds to TTR amyloid fibrils, which is useful to differentiate between the different types of amyloidosis, showing intense marking in ATTR amyloidosis and none in AL.3 Therefore, it is useful to differentiate ATTR from AL cardiac amyloidosis when a monoclonal protein is not identified by serum kappa/lambda free light chain ratio analysis or serum or urine protein immunofixation. A patient with strongly positive radionuclide imaging, associated with an absence of a plasma cell dyscrasia, is highly specific for ATTR cardiac disease, so tissue biopsy is not required.10
Confirming the diagnosis is with an endomyocardial biopsy, which is 100% sensitive. In this case, given the suspicion of cardiomyopathy of unknown origin. In the context of heart failure with echocardiographic signs of restriction and coronary angiography without lesions, it was decided to perform the procedure, which helped to establish the diagnosis. The biopsy result is also relevant because it differentiates amyloidosis from other types of infiltrative diseases and the specific type of amyloidosis, each with a specific treatment and prognosis.11 There is also the possibility of performing a biopsy of other tissues, such as the subcutaneous fat of the abdominal wall. Nevertheless, this has a low sensitivity (14%) in ATTRwt amyloidosis, which can lead to false negatives.3
In patients diagnosed with cardiac ATTR amyloidosis, noninvasive sequencing of the TRR gene is useful for discriminating between wtATTR and hATTR. It is used in patients diagnosed with cardiac ATTR amyloidosis. Thus, diagnosis of hATTR allows for genetic counseling and disease-specific treatment.12 However, it was not performed because our patient had already died.
In the case described, no other triggers for acute decompensation were found. The blood pressure was controlled. The ventricular rate was normal, no ischemic cause was found, and there were no associated infections. The patient had good adherence to medical treatment, the paraclinical tests did not show metabolic alterations, and there was no different valvular or mechanical dysfunction that would explain the symptoms; thus, amyloidosis is attributed to the causal link of acute heart failure in this patient.
The main treatment and prognosis depend directly on the type of amyloidosis; thus, AL amyloidosis has a haemato-oncology-directed treatment with specific chemotherapeutics and usually has a worse prognosis.13 On the other hand, ATTR amyloidosis is based on the management of heart failure with emphasis on the treatment of water overload, in addition to new therapies aimed at blocking or stabilizing the transthyretin tetramer or clearing the amyloid fibers. Recently, Tafamidis has shown a decrease in cardiovascular hospitalizations, 30-day mortality, and functional decline. The incidence of adverse events is similar in the tafamidis and placebo groups.14 It stabilizes transthyretin tetramer by binding to the thyroxine-binding site of TTR tetramer and preventing dissociation of TTR tetramer into monomers, thus reducing TTR amyloid.15
For the treatment of hereditary TTR amyloidosis, new therapies have been released. Among these new therapies are ribonucleic acid (RNA)-targeted therapies that interfere with hepatic TTR synthesis (patisiran, inotersen, and vutrisiran). Diflunisal is a nonsteroidal anti-inflammatory drug that can stabilize the TTR tetramer in vitro and may prevent the formation of amyloid deposits in the heart.16 Patisiran is approved for the treatment of peripheral nerve disease caused by hereditary transthyretin amyloidosis, and it may halt the progression of the cardiac manifestations of hATTR.17 Inotersen is indicated for polyneuropathy of hATTR and benefits have also been shown for amyloid cardiomyopathy.18 Organ transplantation is a definitive treatment option for patients with severe cardiac amyloidosis. Heart and liver transplantation has been used to treat hATTR; for AL amyloidosis, a heart transplant is recommended.12
Other authors, such as Bodard et al. in France, Franco et al. in Barcelona, and Fernandes et al. in Portugal report cases of acute heart failure due to amyloidosis similar to this one. They report elderly patients (93, 90, and 78 years old, respectively) with acute congestive heart failure symptoms and electrocardiographic and echocardiographic findings like this one, in whom amyloidosis was found by a salivary gland or subcutaneous fat biopsy.19-21 The case’s novelty is that no similar cases were found in Latin American populations.
Conclusions
Amyloidosis is an underdiagnosed multisystemic pathology due to its wide range of symptoms. We present a case of an elderly patient who debuted with acute heart failure. Amyloid infiltration was documented in echocardiography and endomyocardial biopsy. It is essential to make a timely diagnosis to offer specific treatment, especially in ATTR, since when this is associated with cardiac involvement, it has a worse prognosis.











 nueva página del texto (beta)
nueva página del texto (beta)


