Introduction
The Mexican College of Interventional Cardiology and Endovascular Therapy (COMECITE for the name in Spanish: Colegio Mexicano de Cardiología Intervencionista y Terapia Endovascular) formed the consensus group with a designated chairman and co-chairman; that later distributed functions to the rest of the members. Every member searched and analyzed relevant publications about fasting, hydration, early discharge, low contrast in percutaneous interventions, monitoring patients in intensive care unit and ward post interventional procedures, and vascular complications settings. The authors used the Cochrane Handbook1 for systematic reviews of interventions and AMSTAR 2 (A MeaSurement Tool to Assess Systematic Reviews): a critical appraisal tool for systematic reviews that included randomized or non-randomized study trials of healthcare interventions.2 The members also reviewed single papers regarding special anatomical conditions. The consensus group discussed each paper in an expert panel format, nominal group technique, and anonymous Dolphy survey.3
The consensus timing process took from Dec 1/2021 through April 2022.
The authorship for publication follows the International Committee of Medical Journal Editors (ICMJE).4
Fasting
The catastrophic event of pulmonary aspiration of gastric contents justifies the usual indication for fasting before general anesthesia to prevent several respiratory syndromes, such as aspiration pneumonitis due to chemical injury after inhalation of gastric contents, aspiration pneumonia for inhalation of oropharyngeal secretions, and pathogenic bacteria colonization, airway obstruction, lung abscess, exogenous lipoid pneumonia, and chronic interstitial fibrosis.5,6
Death or permanent severe injury may result from pulmonary aspiration during general anesthesia, and the major risk factors are, in order of frequency.7
Emergency procedures.
Acute intraabdominal processes.
Morbid obesity.
Gastroesophageal reflux disease.
Diabetes mellitus.
Recent oral intake.
Recent opioid administration.
Major trauma.
Previous gastric bypass or sleeve.
Neurological disease.
Pregnancy.
The American and Canadian Anesthesiologists recommend eight hours of fasting from fatty food or meats, six hours for non-human milk or a light meal, four hours for breast milk, and two hours for clear liquids before the anesthesia. However, the Canadian Pediatric Anesthesia Society and the European Society of Anaesthesiology preoperative guidelines are more permissive for less prolonged fasting times, especially encouraging liquids intake.
Anesthesiologists have enough imaging skills to incorporate gastric ultrasound, which is feasible in obese, pregnant, and pediatric patients, especially during the uncertainty of prandial status and gastric emptying. <1.5 mL/kg of clear fluid is consistent with a state of fasting, in contrast with ≥1.5 mL/kg of clear fluid or solids.8
Interestingly, doctors indicated caloric liquids three hours before surgery during the early 19th century to prevent aspiration during anesthesia; one hundred years after, practice switched towards fasting from midnight under the misconception to reduce such risk.9
Anxiety, dehydration, postoperative nausea, hypoglycemia, hypovolemia, and vomiting may result from prolonged fluid fasting indeed, free fluids before anesthesia may reduce postoperative nausea and vomiting, considering no more than 3.5-hour clearance for both clear and non-clear liquids and less than two hours when not exceeding 220 kcal; the same applies for pediatrics.10 Stress response to trauma induces insulin resistance, which associates with a poor prognosis.11
Prolonged fasting causes undesirable effects and does not guarantee stomach emptying; hence the currently preferred indication for liquids by mouth 2 hours before surgery and one hour in pediatrics.12
There is no evidence-based support for fasting before cardiac catheterization, especially for local anesthesia and mild sedation, and there is a lack of supportive evidence for lung aspiration in emergencies, such as percutaneous coronary intervention or brain interventions.13-15 Heart disease patients may also receive poor hydration and diuretics, thereby increasing the risk of contrast dye kidney damage.16,17
The contemporary indication for free feeding before percutaneous endovascular intervention recently received supporting concepts from leaders of the Society for Cardiac Angiography & Interventions (SCAI), perhaps leaving some restrictions for large catheter size-based and valvular procedures.18
Highlighting fasting at any age and gender:
Results from customs, not science.
Usually results in uncontrolled and prolonged periods without meals and liquids.
Associates to morbidity and mortality.
May aggravate poor hydration and exposure to contrast dye kidney damage.
It does not prevent efficiently pulmonary aspiration.
Short-term calorie-liquids:
May prevent nausea and vomit.
Causes faster gastric emptying.
It does not relate to pulmonary aspiration.
Finally, free-feeding before cardiac catheterization (not general anesthesia, large catheter size, and valvular intervention) may be better than fasting.
Patients must have a fasting protocol based on individualized needs under personal schemes or institutional program centers for the best patient comfort and safety. Nonetheless, the Consensus group decided on the following recommendations, applied to all ages, genders, and procedure complexity:
Indications for fasting do not apply to immediate urgency for cardiac catheterization and rescue interventions that proceed without any delay.
Encourage the anesthesiologist to perform ultrasound identification of the contents of the antrum; this helps not to delay the procedure when the fasting time is uncertain.
Avoid more than twelve hours of fasting. The usual indication for «nothing by mouth (NPO)» since dinner, for institutional schedule, forces at least twelve hours fasting for the first-time procedure chart and increases on the second time and so on with the rest of the patients, being up to more than 20 hours for the afternoon procedures. Overcome the problem with the following:
Indicate NPO since dinner on all patients.
Indicate at 08:00 AM.
Indicate at 08:00 a liquid diet with glucose for the rest of the morning patients.
-
Indicate complete breakfast and liquid diet at noon for all scheduled afternoon patients (Table 1).
Table 1: Fasting summary.
Coronary, structural or peripheral Fasting hours Previous diet Recommendations Post-intervention meal Emergency None No matters Gastric ultrasound As soon as possible Morning 1st time 8 No restricted dinner — As soon as possible Morning 2nd time 8 No restricted dinner ≤ 200 clear liquids at 08:00 As soon as possible Morning 3rd time 8 No restricted dinner ≤ 200 liquids with ≤ 220 kcal at 08:00 As soon as possible Afternoon 1st time 6 No restricted breakfast — As soon as possible Afternoon 2nd time 6 No restricted breakfast ≤ 200 clear liquids at noon As soon as possible Afternoon 3rd time 6 No restricted breakfast ≤ 200 liquids with ≤ 220 kcal at noon As soon as possible Assess every patient’s hydration status for additional parenteral fluids.
Consider shorter fasting periods before low catheter-sized procedures under local anesthesia and mild sedation.
Above recommendation improves significantly on individualized care and indication for fasting.
Pediatrics must avoid more than six hours of fasting and consider two-hour clear liquids before the procedure.
Consider the patient’s consciousness condition for the next meal after the procedure, which should be sooner.
Hydration
Hydration is essential for metabolism, substrate transport across membranes, cellular homeostasis, temperature regulation, and circulatory function. Normal plasma osmolality ranges 266-301 mOsm/kg may be considered normal but is age-dependent. Inter-individual differences and comorbidities are the primary reasons why widespread consensus regarding the daily water requirements has not been reached to this date.19
The 2004 US National Academy of Medicine (NAM) publication, which presented dietary reference intakes for water, this report concluded that: (a) individual water requirements can vary greatly on a day-to-day basis because of differences in physical activity, climates, and dietary contents; and (b) there is no single daily water requirement for a given person.20
Optimal hydration must be a premise to avoid complications in interventional procedures. Pre- and post-intervention hydration balance is the best method to prevent contrast-induced nephropathy (CIN) plus low contrast volume. Patients with dehydration or low cardiac output need a high fluid volume infusion but are restricted for congestive heart failure or high cardiac output and must individualize hydration.
Initial hydration evaluation starts with clinical symptoms and signs of dehydration like thirst, dry mouth, low volume of urine or sweat, dark-colored urine, dry skin, feeling tired, and dizziness in adults; dry mouth and tongue, crying without tears, no wet diapers for three hours or more, high fever, sleepy or drowsy, irritability, eyes sunken in infants and young children. Upgrade dehydration includes confusion, fainting, lack of urination, tachycardia, tachypnea, and shock.
Methods for assessing hydration include hematocrit, plasma, urine, saliva or tear osmolarity, serum sodium, bioimpedance, body mass, vital signs, and hormone variables. Precision, reliability, cost, invasiveness, and required time rates from low to high and often impractical. Ultrasound is a useful tool for evaluating inferior vena cava diameter with a normal value of 50%, suggesting a normal mean right atrial pressure (RAP) of 0 to 5 mmHg. Lack of vena cava collapse suggests elevated mean RAP 10-20 mmHg. It is worth the invasive central venous pressure monitoring or Swan Ganz catheterization and lactic acid test.
Optimal hydration in percutaneous endovascular intervention is essential to maintain homeostasis and reduce risk complications in widespread scenarios and structures (coronary, structural, and peripheral).21-23
Intravenous hydration with 0.9% saline solution 1 to 1.5 mL/kg/min infusion rate is a conventional hydration technique that should be applied pre, peri, and post-procedure, starting 12 hours before the intervention and continuing for up to 12 hours after completion of the hemodynamic procedure achieving the goal of a urinary flow of 150 mL/h.24,25
Central venous pressure (CVP) using a venous catheter at a superior cava level provides records to guide the liquids handling; consider that pericardial, intra-abdominal, and intrathoracic pressure may modify the CVP. The normal value of CVP is 8 to 12 cmH2O or 1 to 8 mmHg, 1 cmH2O is equal to 0.735591 mmHg. Values below the lowest normal ranges indicate volume needs26 and values greater than the highest range indicate fluid overload.
Swan Ganz catheter provides useful information on body hemodynamics in unstable patients and should be the gold standard for hydration monitoring, yields pulmonary artery pressure, CVP, pulmonary capillary wedge pressure, cardiac output, mixed venous oxygen saturation (SvO2), systemic vascular resistance, pulmonary vascular resistance, and cardiac index.27,28 POSEIDON trial concludes that left ventricular end-diastolic pressure (LVEDP) guides hydration in patients with GFR < 60 mL/min/1.73 m2 by MDRD equation and diabetes, age > 77, hypertension, and history of CHF, reducing major adverse event, death, and dialysis in contrast nephropathy status. Scale protocol recommends a pre-procedure saline infusion rate of 3 mL/kg/h, during the procedure with LVEDP 18 infusion rate of 1.5 mL/kg/h. Post-procedure infusion rate continued for 4 hours at least.29 Hydration governed by urinary volume (RenalGuard) has a console with software that measures the urinary volume excreted hourly and replaces intravenously the same amount per hour.30
Recommendation
With comorbidities, always estimate hydration status in pre-and post-percutaneous endovascular procedures.
Clinical examination findings and urinary flow may be a hydration guide in stable patients.
Parenteral solutions and rates could be evaluated based on serum or urinary osmolality, by ultrasound assessing inferior vena cava collapse index, and invasive through central venous pressure, or Swan-Ganz catheterization.
In patients with contrast nephropathy risk, LVEDP guides hydration solutions rate.
Indicate invasive monitoring tailored hydration.
Ultra-low contrast in percutaneous intervention
Percutaneous intervention procedures use intravascular iodinated contrast media injections. The maximum allowable volume of iodine contrast in healthy adult individuals is ≤ 300 mL with a 300 mg I/mL concentration. In patients with renal insufficiency, the contrast volume should be as low as possible, not exceeding 5 × weight (kg) / creatinine (mg/dL) with a 300 mg I/mL concentration.31
Acute renal failure is a common complication in interventional procedures; it may increase morbidity, mortality, and healthcare cost.32 Contrast-induced nephropathy (CIN) may appear after 48-72 hours from exposure to a contrast agent with an increase of serum creatinine values ≥ 0.5 mg/dL or at least ≥ 25% elevation compared to baseline.33 It particularly affects subjects with chronic kidney diseases (CKD), diabetes, heart failure, acute coronary syndromes, and cardiogenic shock. Intra-arterial vs intravenous contrast media administration has a greater risk of CIN, although the mechanism of this phenomenon is not clear.34
Ultra-low contrast coronary angiography is a technique performed using less than 15 mL of nonionic, iso-osmolar contrast agent volume per estimated glomerular filtration rate (eGFR), a ratio should be less than 1 (e.g. if the patient’s eGFR equals 15 mL/min/1.73 m2, the CV should be less than 15 mL). Consider using 5-6F catheters without side holes, and small syringes (e.g., 3 or 5 mL); 3 mL is sufficient to visualize the left coronary artery, whereas 2 mL is enough for the right coronary artery. Remove contrast dye before any drug administration (e.g., nitroglycerine) or when exchanging catheters to avoid pushing it into the patient.
Acquisition time with a high frame rate (i.e., 30 frames/s) helps. Spider view and cranial right anterior oblique projection may be enough to visualize the left coronary artery lesion location, and cranial left anterior oblique projection are usually sufficient for right coronary angiography anatomy. If more projections are necessary, contrast dilution with saline 2:1 may limit the overall contrast amount. Biplane angiography limits the number of acquisitions.35 Patients with renal disease usually have calcified lesions in proximal coronary artery segments which may help to identify the ostial and facilitate catheter engagement. To confirm the proper engagement, inject 10-20 mL of saline through the catheter and observed temporal changes in the electrocardiogram like T-wave inversions or ST-segment depression or elevation.36 This method requires heparin administration and has some risk of coronary dissection.
Hydration remains the cornerstone of CIN prevention; in the POSEIDON trial, left ventricular end-diastolic pressure (LVEDP) guided fluid administration in patients undergoing cardiac catheterization. According to this study, each patient should receive a saline infusion of 3 mL/kg one hour before the procedure. Then, the fluid rate administration adjusts to LVEDP, i.e., 5 mL/kg/h for LVEDP lower than 13 mmHg, 3 mL/kg/h for LVEDP 13-18 mmHg, and 1.5 mL/kg/h for LVEDP higher 18 mmHg. The fluid rate starts at the beginning of the procedure and continues during the procedure and for the next four hours.37
Invasive physiological assessment of coronary lesions with fractional flow reserve (FFR), instant wave-free ratio (iFR), or another interchangeable method should tailor the intervention. Image evaluation with intravascular ultrasound imaging (IVUS) or optical coherence tomography (OCT) which use a mixture of saline and colloid or dextran-40 highlights the feasibility of interventional procedure success.
Pre-dilatation of the lesion is permitted if the imaging catheter does not cross the evaluated lesion. The use dynamic coronary Roadmap system (DCR) with Azurion (Phillips Healthcare), is a novel technology that creates dynamic motion-compensated real-time coronary arteries, which reduce the fluoroscopy time and contrast volume; this novel system is safe and effective.38
Early discharge after percutaneous coronary interventions
Early discharge is a service physicians and healthcare professionals provide for patients’ home care, including treatment and supervision after percutaneous interventions (PCI). It could be feasible and safe.
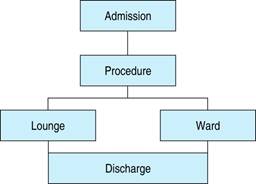
There are two types of early discharge, 1. Same-day discharge39-42 or 2. early discharge (24 to 72 hours).43,44 For the first group, the hospital would have lounge facilities or a similar hospital area. For the second group, the hospital ward is enough (Figure 1).
The lounge is a dedicated facility area able to host patients with individual cubicles, comfortable seats with or without massage, monitor TV and entertainment devices, fast Wi-Fi, restrooms, and shower facilities; should have vital signs monitors (ECG, blood pressure, temperature, oxygen saturation) and adjacent resuscitation room. The staff team includes nurses and physicians trained in standard and emergency procedures. 12-lead ECG and laboratory are also available. Fed and drinks were allowed 30 minutes after the patient’s arrival. Open working hours of the lounge starts at 08:00 and closed at 20:00; the patient long monitoring stay would be less than 6 hours.45
Diagnostic or interventional procedures such as radial or femoral vascular approaches46 are suitable for early discharge, but a stable patient is essential.
There are two main types of active arterial closure devices: 1. collagen plug (e.g., AngioSeal or Exoseal), which uses suture cinches or/and collagen plug, and the anchor breaks down over months. 2. Suture-mediated (e.g., Perclose), uses a suture not absorbable on each side, tied using a preloaded knot, the suture is cut close to the arterial wall, and active closure method involving surgical staple/clip technology (e.g., StarClose) which uses not absorbable clip deployed through the peel-away sheath.47
The exclusion criteria for early and same-day discharge are: cardiac arrest, shock, complicated acute myocardial infarction, congestive heart failure, urgent or emergent procedure, left main intervention, complex lesions, large volume contrast dye (> 500 mL), decreased renal function (eGFR 80, > 30 km from PCI facility,48 left ventricular ejection fraction < 45% or right ventricular fractional area change < 35%, and use of GP IIb/IIIa inhibitors.
Early discharge 24-72 hours, exclusion criteria are unstable patients, cardiac arrest, shock, acute myocardial infarction complications, congestive heart failure, and acute renal failure.
Candidates for early discharge checklist (Table 2).
Table 2: Discharge checklist.
| Parameter | Normal |
|---|---|
| Alert status | ✓ |
| Vital signs | ✓ |
| Capillary refill | ✓ |
| Oral tolerance | ✓ |
| Urinary flow | ✓ |
| Hydration | ✓ |
| No vascular sheaths or catheters | ✓ |
| No bleed or bleeding risk | ✓ |
| No hematoma | ✓ |
| No pain, distal pulse, and temperature | ✓ |
| No side effects | ✓ |
| No interaction medication | ✓ |
| No arrhythmias | ✓ |
| No significant abnormal lab test | ✓ |
| No significant electrocardiogram baseline changes | ✓ |
| Normal walking tolerance | ✓ |
After discharge patient, relatives or caregiver informs to hospital medical staff or Cardiologist in charge of any clinical change or complaints to a 24 hours phone number.
Post-interventional procedure monitoring in the hospital ward
Hospital Ward is a screening or recovering area where patients should be monitored and studied closely.
The inclusion criteria are diagnostic, peripheral, coronary, or structural percutaneous interventions without serious complications or arterial or venous sheaths at the site.
The hospital ward staff must have the medical records and complete procedure information after every intervention.
Initial evaluation encompasses AVPU score (alert, verbal response, painful response, or unresponsive)49 vital signs (heart rate, respiratory rate, systolic and diastolic pressure, temperature, oxygen saturation), capillary refill, appetite, urinary flow, hydration, and bleeding, which would be recording every 4 hours.
Monitoring issues from initial admission to discharge:
Bleeding with compression devices or conventional compressive dress supervision and hypovolemic shock evaluation.50
Evaluate local pain, distal pulse, and temperature.
Supervise hydration (clinical and urinary flow) with an intravenous solution.
Monitor acute and chronic arrhythmia.
Confirm oral tolerance and appetite.
Side effects evaluation and classification (serious or not) inherent to the procedure, medications, or allergic reactions.
Medication interaction checker.
Supervise walking tolerance after the procedure.
Consider laboratory, electrocardiogram, X-ray, echocardiogram, computed tomography scan, or magnetic resonance as required.
Percutaneous post-interventions in hospital ward discharge must be limited to a checklist (Table 2).
Post-interventional procedure monitoring in the Intensive Care Unit
The Intensive Care Unit (ICU) is a department of a hospital in which unstable patients are kept under constant observation and support for failing vital functions.51
Inclusion criteria are diagnostic, peripheral, coronary, or structural percutaneous interventions with pre or post-procedure complications such as cardiac arrest, shock, complicated acute myocardial infarction, congestive heart failure, pulmonary embolism or edema, life-threatening cardiac arrhythmias, urgent or emergent procedure, left main intervention, complex lesions or procedure, large volume contrast medium > 500 mL, decreased renal function (eGFR <60 mL/min) or acute renal insufficiency, left ventricular ejection fraction < 45% or right ventricular fractional area change < 35%, and use of GP IIb/IIIa inhibitors.52
After the intervention, the medical record of the patient and complete procedure information should be provided to the ICU staff.
Initial evaluation encompasses AVPU score (alert, verbal response, painful response, or unresponsive),50 airway, vital signs (heart rate, respiratory rate, systolic and diastolic pressure, temperature, oxygen saturation), capillary refill, bleeding with compression devices or conventional compressive dress, vascular sheaths, catheters or leads. Local pain with distal pulse and temperature or organ site pain, hydration (clinical and urinary flow), intravenous solution, oral tolerance, and appetite. Arrhythmias with acute or chronic onset, side effects, and classification (serious or not) inherent to the procedure, medications, allergic reactions, and medication interaction checker, would be assessed and recorded every hour.
Central venous pressure (CVP) and Swan Ganz catheter provide useful information on body hemodynamics in unstable patients and should not be withdrawn until the patient is stable.
Lab tests, electrocardiograms, X-ray, echocardiograms, computed tomography scans, or magnetic resonance would be individualized and performed as required.
Anaphylaxis score53 and cardiac shock classification54 should be evaluated constantly from initial admission to discharge.
Shock treatment was established as the etiology was identified.55 The hemodynamics type shock parameters must guide the specific treatment (Table 3).
Table 3: Hemodynamics type shock parameters.
| Type of shock | mPAP echo | CVP | MAP | PCWP | CO/SV | SVR | DO2I |
|---|---|---|---|---|---|---|---|
| Normal range | < 25 mmHg | 8-12 cmH2O | > 60 mmHg | 4-12 mmHg | 2.5-4.0 L/min/m2 | 700-1,500 dynes/s/cm-5 | 500-600 mL/min/m2 |
| 1-8 mmHg | 33-47 mL/m2/beat | ||||||
| Hypovolemic | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ |
| Cardiogenic | ↑ | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ |
| Obstructive | ↑ | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ |
| Distributive | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↑ |
mPAP = mean pulmonary artery pressure. CVP = central venous pressure. MAP = mean arterial pressure. PCWP = pulmonary capillary wedge pressure. CO/SV = cardiac output/stroke volume. SVR = systemic vascular resistance. DO2I = global oxygen delivery index. mPAP echo = tricuspid regurgitation peak velocity2 × 4 (0.61) + 2. DO₂I = Q × (Hb × SaO2 × 1.34 + (PaO2 × 0.003)/m2SC.
Post-interventional percutaneous procedure discharge ICU criteria have been established when the patient at the time is stable with no serious complications nor arterial or venous sheaths at the site. Avoid discharging patients from ICU after 19:00.56
Vascular access complications
The increased use of arterial radial access diminished vascular complications compared to the femoral approach,57 even so, femoral access is necessary for structural, peripheral, and coronary high-risk patient procedures.
Bleeding, ecchymosis, hematoma, pseudoaneurysm, infection, distal ischemia due to embolic occlusion, on-site or distal dissection, arteriovenous fistula, compartment syndrome, and perforation are common complications with any vascular intervention. Compartment syndrome is the most serious complication, frequently related to trauma with the guidewire accompanied by a forearm, leg, or pectoral hematoma, which increases pressure in the compartment area and potentially damages the muscle and nearby nerves.
Radial and ulnar artery
Spasm is their more frequent complication, conditioning catheter/sheath entrapment, and eversion endarterectomy; obey to small vessel diameter, insufficient sedation/analgesia, and repeated punctures. Topical, subcutaneous, or sublingual nitroglycerin, reactive hyperemia with blood pressure cuff inflated to 30 mmHg above-average systolic pressure for 3 minutes, forearm heating (i.e., Balbay maneuver)58 for 3 minutes, hydrophilic sheaths or sheathless guide catheters, excessive catheter manipulation avoidance, telescopic technique use, intraarterial verapamil 2.5 mg, diltiazem 2.5-5 mg or nicardipine and nitroglycerin 10-200 mcg and sedation/analgesia, may prevent spasm.59
Femoral artery
The inguinal hematoma is the most frequent complication that may happen sooner or later, imposes a potential risk for the patient, and increases cost due to delays in discharge and ambulation. Over-anticoagulation, large-diameter sheaths, obesity, and female gender are the more common risk factors for hematoma, mostly prevented with refined puncture techniques and vascular closure devices; conservative treatment is the best initial option.
Retroperitoneal hematoma is one of the most serious complications, usually after the iliac or femoral vessels lesion with bleeding into the retroperitoneal space that may hold a large amount before detection. Repeat puncture attempts, over-anticoagulation, large introducer diameters, concomitant venous sheaths, obesity, low body weight, peripheral vascular disease, renal failure or elevated creatinine, hypotension or shock, low platelet count, prolonged procedure duration, repeat PCI, arterial puncture site, and female gender, low body surface, and older age are the more common risk factors, better prevented with a single arterial wall puncture, micropuncture technique,60 fluoroscopic and or ultrasound guidance, reverse over-anticoagulation, arterial closure device, adequate vessel hemostasis, and supervised compression.61
Venous access
Cannulation of antecubital, internal jugular, subclavian or femoral vein is feasible for catheters or leads and allows for high fluid administration, monitoring, or pacing. Ultrasound guidance and or landmark techniques facilitate structure identification. Trendelenburg’s position may prevent air embolization. Complications are arterial puncture, hematoma, hemorrhage, embolism (air or catheter fragment), erosion or perforation, infection, phlebitis, and thrombosis.62
Recommendations to prevent and treat complications:
Master the technique.
Try a single wall puncture.
Use ultrasound guidance puncture in difficult cases.
Be cautious and get enough material, including hydrophilic wires and use the smallest sheath diameter.
Perform a checklist before the procedure with available materials and medications.
Verify for enough gadgets to manage possible complications.
Stay calm and, if necessary, ask for help from more experienced staff.











 nueva página del texto (beta)
nueva página del texto (beta)