Introduction
Cardiac tumors can be conceptualized as primary simple, primary complex and secondary. Primary neoplasms of the heart are uncommon; they occur at an incidence rate of 30 per 100,000 people per year, of which 80 to 90% are benign.1,2 Being cardiac myxomas the most common of them, accounting for 50% of all benign cardiac tumors in adults. Finally, secondary tumors are 30 times more common than primary cardiac tumors. Myxoma is derived from its appearance as a cell poor myxoid neoplasm with a mucopolysaccharide rich extracellular matrix. They may present with the triad of obstructive cardiac symptoms including dyspnea, presyncope and sudden cardiac death;1 embolic and constitutional symptoms. However, some studies report dyspnea as the most common symptom of cardiac tumors.2
The most common location is the left atrium (75%), then the right atrium (15%), with the remaining cases equally distributed between the right and left ventricle.1 Tumors of the right side grow suspicion of metastatic disease.
There is a possible association between reports of lymphoproliferative neoplasms within a cardiac myxoma with Epstein-Barr virus (EBV) infection and inflammatory cytokines. We present two different cases of right atrial myxoma.
Case presentation
Case 1: a 59-year-old man, without a positive family history, under ten years follow up for cutaneous T cell Lymphoma, presented with progressive dyspnea of nine months of evolution. A physical exam revealed a mitral regurgitation murmur.
The electrocardiogram showed sinus rhythm, p-mitral and non-significant ST-segment depression.
A transthoracic echocardiogram (TTE) reported a mobile right atrial mass (Figure 1) with right lateral pedicle anchored to the right atrial wall near the origin of the inferior vena cava without compromise of venous drainage. No vascularization evidence by color Doppler was found, neither obstruction nor infiltration to the right ventricle.
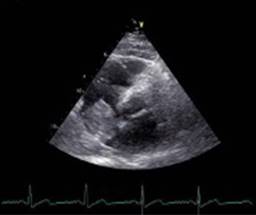
Figure 1: Transthoracic echocardiogram showing a right atrial mass of 42 × 49 mm of diameter with heterogeneous echogenicity and areas of calcification.
A trans-esophageal echocardiogram reported severe mitral regurgitation with P3 segment prolapse and a regurgitant jet area of 5.6 cm2. The coronary angiography showed a tumor nutrient artery emerging from the posterolateral branch of the right coronary artery (Figure 2). The patient underwent right atrial tumor resection through right atriotomy and mechanical mitral valve replacement of 31 mm through a transeptal approach. The right atrial pedicle tumor of 25 × 30 mm was infiltrated into two different locations: the inferior vena cava outflow tract and the right atrium wall (Figure 3). The pathologic study reported atrial myxoma and fibromyxoid degeneration of the mitral valve (Figure 4). Surgery was carried out without complications, and post -operative care was unremarkable. He was discharged satisfactorily after anticoagulant adjustment, staying free from recurrences.
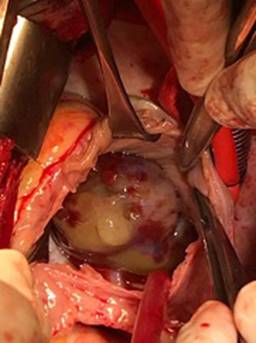
Figure 3: In situ, operative view of right atrial, multilobulated mass with bleed areas, consistent with atrial myxoma.

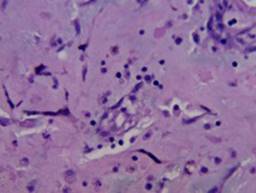
Figure 4: 40× H&E staining showing myxoid matrix. There is typical ring structure infiltration by chronic inflammatory cells. No mature vessels are seen, and neither are interconnecting anastomosing channels. No evidence of cutaneous T cell lymphoma was found.
The patient did not go into remission of cutaneous lymphoma. Two months after surgery, he developed reactivation of dermatological lesions in the left pelvic limb. However, a contrast abdominal CT showed no evidence of lymphoproliferative activity. He continued management with pegylated interferon, deflazacort, hydrocortisone, topic fluocinolone and symptomatic management with hydroxyzine and ursodeoxycholic acid.
Case 2: a 61-year-old woman without any history of chronic degenerative diseases developed one month before admission left internal jugular vein and subclavian vein thrombosis. The patient was started on anticoagulation with rivaroxaban for six months.
Ten days before her admission, the patient presented sudden dyspnea. Pulmonary thromboembolism was initially suspected, and pulmonary angiography was performed, which revealed an apparent intracavitary thrombus in the right ventricle. The patient was sent to our unit for further evaluation.
On admission, a hypoechoic thrombus in the basilic vein, cephalic vein and right jugular vein with collateral venous vessels that flow into the left jugular vein was confirmed with a new ultrasound.
A transthoracic echocardiogram was performed, which showed an 80 × 40 mm cardiac mass in the right atrium, with insertion at the interatrial septum, heterogeneous, with mobile calcifications that protrude into the right ventricle occupying it entirely. The left ventricle had normal global and segmental mobility with LVEF of 75%.
A contrast CT of the thorax and abdomen (Figure 5) revealed a heterogeneous mass measuring 55 × 44 mm, with partial extension to the RV outflow tract. Pretracheal and aortopulmonary adenomegaly was present. However, no data of a primary tumor was found. Our principal differential diagnoses were myxoma and angiosarcoma.
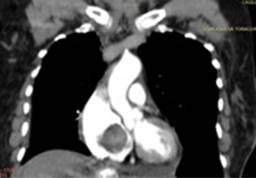
Figure 5: Contrast CT scan showing a mass in the RA and RV, predominantly hypodense, heterogeneous, with no significant enhancement after contrast. Seated on the interventricular septum, occupying the tricuspid entry point and partial extension to the RV outflow tract. No calcifications.
The patient underwent resection of the mass (Figure 6). In the histopathological report, a cardiac myxoma with the greatest diameter of 7.5 cm was reported (Figures 7 and 8). The surgical border was free of the lesion. The patient was weaned off mechanical ventilation without complications but presented a hypertensive emergency at admission to the post-surgical unit that was successfully treated with nitroprusside. Prerenal acute kidney injury improved with crystalloids. The patient was discharged nine days after surgery.
Discussion
Primary heart tumors are rare, found in 0.001 to 0.3% of unselected patients at autopsy.1 About 75% of primary tumors are benign, and 50% of these are myxomas, which most commonly arise from the left atrium.3,4 Only 15-20% of myxomas arise from the right atrium, in the interatrial septum close to the border of the fossa ovalis.5 Sporadic cases arise as isolated and solitary left atrial tumors, whereas familial myxoma can occur in any cardiac chambers as a multicentric neoplasia.6 Myxomas range in size from 1 to 15 cm and are oval or lobulated with a smooth surface.
Atrial myxomas occur predominantly in females, between the fourth and sixth decade of life,7 with a 2:1 female preponderance.
Approximately 10% of myxomas follow an autosomal dominant genetic pattern, as in Carney’s complex, which presents as atrial, cutaneous and mammary myxomas, lentigines, blue nevi, endocrine disorders and testicular tumors. Other myxoma syndromes include LAMB (lentigines, atrial myxomas, mucocutaneus myxomas and blue nevi), NAME (nevi, atrial myxomas, myxoid neurofibroma, and ephelides).8
Most tumors of the right side are metastatic: in order of frequency, primary lung cancer, breast cancer and hematologic malignancies.4 In the first case, metastasis was the first diagnostic impression, although no systemic manifestations such as fever, anemia, leukocytosis and weight loss were reported.
Most patients present with one or more symptoms of the triad of embolic events, intracardiac flow obstruction and constitutional symptoms.9 Obstruction and dyspnea are the most common manifestations.2,5 Small myxomas may go asymptomatic (about 10%),5 as symptoms depend on size, location, mobility, physical activity and body position. Functional tricuspid stenosis may be found and, a «tumor plop» early in diastole has been reported.7 Concomitant signs of mitral or tricuspid valve insufficiency are described.6
The tumor’s long stalk may obstruct the orifice of the tricuspid valve resulting in syncope or sudden death.4
Up to 90% may develop systemic symptoms and occur independently of tumor size and location and include physical weakness, lethargy, fatigue, loss of appetite, anorexia, recent and progressive decrease in body weight and persistent and unexplained low grade fever. Arthralgia, myalgia, facial edema, hyperhidrosis and nocturnal hemoptysis and chronic anemia are less common. Paraneoplastic manifestations may occur, such as vasculitis, Raynaud phenomenon, amyloidosis, livedo reticularis, depletion of factor VII and others.6,10,11
As was shown by the second case, embolism may be a presenting sign in up to 30% of patients. The contour of the tumor is most often lobular and smooth but can be villiform in appearance, which is believed to be associated with thromboembolism.2 They are caused by tissue fragmentation, detachment of tumor as a whole and dissemination of overlaying thrombi. It should be clear that venous thrombosis has no relation to arterial thrombosis.
Embolism is an important complication occurring in 30-50% of patients with cardiac myxomas and is associated with cardiac mortality, especially preoperative. Irregular surface contributes to tumor fragmentation and this was found to be an important risk factor contributing to embolism (HR 2.7); also, a high platelet count has been associated with an elevated risk of thrombosis. Inflammatory cytokine levels increased in patients with cardiac myxomas, which may be related to a high platelet count.12,13 Prompt surgical excision of myxomas is indicated for patients at increased risk of embolism. Venous thrombosis associations with atrial myxomas are very broad. Indeed, an association between atrial myxoma, Budd Chiari syndrome and portal vein thrombosis has been described.14
Predictors of embolism are tumor size. Tumor size above 4.6 cm predicted embolism with a sensitivity of 77% and specificity of 73%, with an area under the curve of 0.858. Left atrium diameter and irregular villous surface morphology were significantly higher in the embolic group.15 Higher BMI, E/e’ ratio and older age were reported in other studies, with a similar ROC of 0.83. Interestingly, patients who developed postoperative atrial fibrillation, stroke or embolism related events in the perioperative period had significant higher CHAD2DS2 VASC scores (≥ 4 points) than those without embolism. However, it is debatable whether embolism occurred due to atrial fibrillation or tumor embolism.16,17
Obstructive heart failure is associated with solid, polypoid tumors, while neurologic and other embolic events represent the most common clinical feature of fragile papillary myxoma.
TTE is useful for detecting the insertion site, morphology, calcifications, cysts, necrotic foci and hemorrhage. Calcification of the tumor is suggestive of myxoma, particularly in the right atrium.3 On echocardiography, a myxoma may be heterogeneous or homogeneous and may have calcifications. Typical findings on contrast-enhanced CT are a spherical or ovoid mass lower in attenuation than surrounding myocardium.2 On CT, heterogeneity is a common imaging feature with areas of hemorrhage, necrosis, cyst formation, fibrosis or calcification. However, the most helpful feature is the narrow base of attachment which is not seen with other neoplasms.15
One of the most difficult differential diagnoses is a thrombus. Axial late gadolinium enhancement magnetic resonance may show small foci of internal enhancement, a useful differentiating feature from thrombus since thrombi do not show enhancement.8
Microbubble contrast agents delineate the myocardium blood interface and may aid in the differential diagnosis. Contrast enhanced ultrasonography is based on microcirculation imaging which can display the blood flow of the mass, which can help differentiate benign and malignant tumors from thrombi. There is no enhancement inside a simple thrombus. Benign tumors have greater enhancement than the myocardial tissue with a mean value of peak enhancement of 0.63; on the other hand, malignant tumors are more inhomogeneous and more strongly enhanced with a mean value of 1.49.18
The study by Kirkpatrick, J et al. demonstrated that enhancement with echocardiographic contrast is useful to differentiate the neo-vascularization of malignancy or vascular tumor from the avascularity of a thrombus. Vascular tumors are hyper-enhanced, while stromal tumors and thrombi are hypo-enhanced. The thrombi failed to enhance.
Continuous infusion of contrast assesses dynamically whether a mass is filled by microbubbles. This intravascular tracer raises the possibility of malignancy.19
There is a possible association between Epstein-Barr virus (EBV) infection, inflammatory cytokines and lymphoproliferative neoplasms with atrial myxomas. A series of 18 cases of primary lymphomas arising within cardiac myxoma have been reported;9 however, none of them is associated with cutaneous T cell lymphoma as in the first case.
It is believed that IL-6 plays a role in the immune-inflammatory response and is a key factor in the B-cell maturation process. IL-6 in atrial myxomas may be associated with the selection of a population of EBV-infected B cells.9
We found the coexistence of mitral insufficiency due to fibromyxoid degeneration and atrial myxoma. This association is not entirely understood.
The treatment of choice is resection, which can be curative. Resection must be performed promptly because of the risk of embolism and sudden death. If pedunculated, it can be easily removed. Resection must be completed with wide margins. Careful manipulation is important to prevent intracardiac implants and recurrence,4 which has been reported in 1-3%. Septal defects due to resection must be repaired; also, adhesion to the leaflets may require valve repair, annuloplasty or valve replacement with a prosthesis. In the first case, we chose a bicaval drainage looking for better exposure of the right atrial chamber, taking advantage of performing a transseptal approach for the implantation of the mitral prosthesis.
There is a survival rate after resection of 92.7% at ten years. However, multicentric myxomas are independently associated with recurrence with an OR = 9.45. In one series of 95 patients, there was only a single recurrence over five years following excision. Patients must be followed with transthoracic echo one year following excision and then every five years.2 After surgical resection of primary lesions, recurrence has been observed in 1-4% of sporadic and 12-22% of familial cases.6
The most common residual effects seen in a small series were neurologic deficits in nine patients. There were four cases of arrhythmias and two cases of mitral regurgitation.16 Both of our patients underwent an uneventful recovery.
Conclusions
Atrial myxoma should be suspected even in unusual locations and regardless of concomitant neoplastic disease. As shown by the first case, there is a possible association with Epstein-Barr virus (EBV) infection, IL-6 and lymphoproliferative neoplasms within the setting of cardiac myxoma. As shown in the first case with severe mitral regurgitation, associated valve disease may be present. The treatment of choice is resection, which can be curative, with a survival rate of 10 years in more than 90%. The second case illustrates the association with embolic events that can be predicted by findings such as left atrium diameter and tumor surface. However, embolic phenomena, as the first presentation, may be misleading. Previously validated scales such as the CHAD2DS2 VASC score may help identify the patients with the highest risk of embolism and post-operative complications.











 nueva página del texto (beta)
nueva página del texto (beta)