Case report
A 25-year-old male came to the emergency room of Hospital Santa Marcelina de Cidade Tiradentes with dyspnea on slight exertion, nausea, loss of appetite and mucous skin pallor. He had jugular turgescence, and his oxygen saturation fluctuated between 85-90%. The symptoms started 02 months ago, with progressive worsening. Expressive enlargement of the right chambers was detected in the two-dimensional transthoracic echocardiogram (TTE), with the anomalous movement of the septum, as well as a heterogeneous echogenic image measuring 66.22 × 55.14 mm (Figure 1). The mass nearly occluded the entire right ventricle from its inlet, with extension through the tricuspid valve (Figure 2), obliterating the flow in some cycles and generating hemodynamic repercussions. The left chambers had no significant abnormalities other than being compressed and flattened by the right ventricle mass (Figure 3). The left ventricular ejection fraction (EF) was 59%, and the pulmonary artery pressure was estimated at 60 mmHg. Due to right ventricular insufficiency, the patient was admitted to the hospital’s Intensive Care Unit and clinically stabilized with dobutmine and small fluid trials. A chest computed tomography was performed, demonstrating mediastinal lymph node enlargement, reaching 24 × 17 mm, beside the right ventricular cardiac mass with 96 mm in its largest diameter, invading the right atrium and pulmonary artery trunk (Figure 4). There was a delayed contrast enhancement of the mass. No signs of neoplasms were found in the contrasted abdominal computed tomography, and deep vein thrombosis was absent in the lower limb venous ultrasonography. As cardiac surgery was not available at the hospital, the patient was referred for evaluation in another service. Sadly, after 37 days of hospital admission, the patient passed away, waiting for cardiac surgery, in a sudden episode of coma, followed by cardiac arrest in pulseless electrical activity. This mass is one of the largest right ventricle masses ever described.1,2
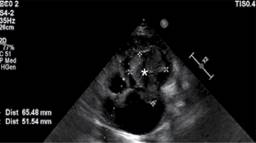
Figure 2: Apical window of the bidimensional echocardiogram revealing a huge mass in the right ventricle, protruding through the tricuspid valve into the right atrium.
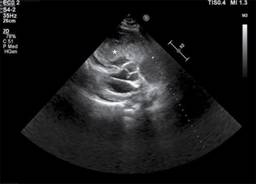
Figure 3: Parasternal long axis revealing huge mass in the right ventricle compressing the left ventricle.
Discussion
According to surgery and autopsy reports, primary cardiac tumors are responsible for 0.3 to 0.7% of all cardiac tumors.3 Benign tumors are approximately 90% of primary cardiac tumors, and 10% are malignant. TTE continues to be the first diagnostic path to evaluate contours, mobility, size, site of attachment and hemodynamic repercussions.4 Employing myocardial contrast during the exam is helpful, since it can provide important information on the perfusion pattern of the mass, differentiating vascular tumors from avascular thrombus, for example.5 Transesophagic echocardiography (TEE) is also fruitful, whereas this technique provides better imaging of structures that could be difficult to evaluate transthoracically. Complementing the investigation with cross-sectional imaging methods, such as computed tomography (CT) and cardiac magnetic resonance (CMR), is very important to establish the extent of disease and better characterize the tumor.6
Amongst the benign cardiac tumors, myxoma is the most prevalent, with nearly half of the benign masses in the adult population.7 They appear as finger-like projections with a smooth surface and areas of calcification, mainly in the left atrium (80%), in individuals between the fourth and sixth decade of life.8 Furthermore, these tumors appear «hypointense» to myocardium on T1-weighted images and «hyperintense» on T2-weighted images in CMR. Most myxomas are sporadic, but they are also associated with a rare familial syndrome, called Carney Complex, caused by defects in the PRKAR1A gene. It is an autosomal dominant syndrome, with multiple endocrine neoplasia and lentiginosis, associated with myxomas of the heart, breast and other sites, in addition to a predisposition to various malignancies.9 Other benign cardiac tumors include fibromas, lipomas, papillary fibroelastomas, cystic tumors of the atrioventricular node and paragangliomas. Rhabdomyomas are the most common benign cardiac tumor in children, accounting for 40-60% of cases.10 Surgical resection of all symptomatic benign cardiac tumors is mandatory, except for rhabdomyomas, since they often spontaneously regress or could be treated with mTOR complex 1 inhibitor.11 Nevertheless, surgical treatment should always be considered, even for small and incidental tumors, especially left-sided and endocavitary lesions, due to embolic risk. Right-sided and asymptomatic tumors without septal defects could be assessed with a strict echocardiographic follow-up.
Malignant cardiac tumors have an exophytic characteristic that distinguishes them from benign tumors. Amongst them, 75% are sarcomas,12 with various types: myxosarcoma, liposarcoma, angiosarcoma, fibrosarcoma, leiomyosarcoma, osteosarcoma, synovial sarcoma, rhabdomyosarcoma (most common in children), undifferentiated sarcoma, reticulum cell sarcoma, neurofibrosarcoma and malignant fibrous histiocytoma.13 They typically present in the right side of the heart and rapidly invade the primary structures, such as valves and walls, obstructing the blood flow. Angiosarcoma is the most common type of malignant cardiac tumor in adults, comprising 40% of the cardiac sarcomas,14 and affecting individuals aged 40-50 years, but have been described in all ages.15 Considering the rarity of cardiac sarcomas, there is little guidance for its treatment. Surgery with negative margins is associated with increased survival and is the mainstay treatment, although complete resection is possible in fewer than half of patients.16 Failure to achieve local control of the disease is the most likely cause of death since metastatic progression tends to appear later.17 Large (> 5 cm) and high-grade tumors are the ones with the highest metastatic potential, and the lungs are the preferred metastatic site.18 A contrasted CT of the thorax should be performed as the principal systemic staging investigation. Cardiac sarcomas typically have devastating consequences and poor outcomes, with a median survival time of fewer than ten months without treatment.19 Indeed, even if the tumor is completely resected, patients frequently develop recurrent disease. Nonetheless, a 34 patient surgical cohort treated at Mayo Clinic over 32 years showed median survival improvement when complete resection was possible (17 × 6 months when complete resection was not possible).20 Adjuvant chemotherapy and radiotherapy brings questionable benefits.
Even though most doctors consider cardiac metastases rare, their incidence ranged from 2.3 to 18.3% amongst all neoplasm detected in autopsy series in the literature. There are few papers published on the subject, but Bussani et al. reported that the tumors that had the highest rate of cardiac metastases were the following, by order: pleural mesothelioma, melanoma, lung adenocarcinoma, undifferentiated carcinomas, lung squamous cell carcinoma, breast carcinoma, ovarian carcinoma, lymphomyeloproliferative neoplasms, bronchoalveolar carcinomas, gastric carcinomas, renal carcinomas and pancreatic carcinomas.21
Cardiac thrombi typically occur in the posterior wall of the left atrium or within the left atrial appendage in patients with atrial arrhythmias. Patients with systolic heart failure often happen to develop thrombi in the left ventricular apex. The occurrence of thromboembolism in the right side of the heart is predominantly related to mobilized deep venous thrombi, especially from the iliofemoral veins. Atrial fibrillation, acute right ventricular myocardial infarction, prosthetic valves, congenital abnormalities, genetic and acquired thrombotic disorders, prosthetic valves and cardiac surgery may also be the cause of right-sided cardiac thrombus.22 Generally, thrombi are serpiginous mobile clots, but they can also present as immobile masses. Treatment possibilities are anticoagulation, thrombolytic therapy and surgical removal of the clots.
The diagnostic imaging exams and clinical data strongly suggest that this patient had a primary malignant cardiac mass, notwithstanding that it was impossible to obtain a biopsy. Unfortunately, the hospital is located in the most poverty-stricken neighborhood of the Greater Sao Paulo area,23 where resources are scarce, such as CMR, and cardiovascular surgery is not easy to perform.
Conclusion
Primary cardiac malignancies are extremely rare and have disappointing outcomes, even when complete surgical resection is performed. The use of echocardiography, CT, and CMR is very important to a better characterization of the lesion and to determine the extent of disease. Patients eventually die from the local progression of the tumor rather than by metastatic invasion. However, clean margin resection can improve survival time and should always be considered.











 nueva página del texto (beta)
nueva página del texto (beta)





