Introduction
Cor triatriatum sinistrum (CTS) is a congenital heart anomaly in which the left atrium is divided into a «posterior superior» and an «anteroinferior» chamber by a fibromuscular membrane that usually contains one or more fenestrations that allow the passage of the blood.1 First described in 1868 by Church2 and its echocardiographic characteristics described by Ostman-Smith in 19843 where they specify that, generally, the chamber into which the pulmonary veins drain is dorsal and obliquely inferior to the chamber of the left ventral atrium with the subdivided membrane bulging upwards and forwards, although this is not always true. Furthermore, the most common site for a single membrane defect was inferior and medial behind the posteromedial commissure of the mitral valve. There is a classification according to Loeffler that divides the CTS into types 1, 2 and 3. Type 1 is characterized by the lack of communication in the membrane itself, type 2 due to having one or more small perforations in the membrane, and type 3 due to a wide opening.4 It represents 0.1-0.4% of all congenital heart malformations.5 It generally involves the left atrium in approximately 83% and rarely the right atrium Cor triatriatum dexter (CTD).6 The clinical manifestations depend on the degree of obstruction of the pulmonary venous return and vary from asymptomatic to pulmonary hypertension.7 Surgical management is indicated in patients with a significant obstruction. This clinical case presents a patient with a complicated urinary tract infection (UTI), de novo atrial fibrillation (AF) CHA2DS2VASc 1 point, and, as an incidental finding on echocardiogram, a CTS.
Case presentation
A 68-year-old male patient, native from Venezuela, mixed-race, have a history of benign prostatic hyperplasia being managed with Tamsulosin 0.4 mg orally every day. The patient is a heavy smoker (pack-year 50), who consulted for one day of evolution of atypical chest pain that began at rest, oppressive, of moderate intensity, not irradiated, lasting 30 minutes, self-limited, associated with palpitations, without vasovagal symptoms. Concomitantly, the patient presented obstructive and irritative urinary symptoms. An electrocardiogram was performed, reporting AF with a rapid ventricular response, administered metoprolol tartrate 100 mg PO single dose, and redirected to our institution. On admission, clinically stable, arrhythmic, tachycardic heart sounds, without murmurs, with pulse deficit, without the presence of congestive or low output signs. The following tests and diagnostic aids were requested (Table 1 and Figure 1).
Table 1: Laboratory tests.
| Hemoglobin | 14.3 mg/dL (13-17) | pH | 7.432 | |
| Hematocrit | 43% (39-52) | pCO2 | 32.9 | |
| Leukocytes | 20.100 (3.600-10.200) | pO2 | 64.3 | |
| Neutrophiles | 89% (45-65%) | HCO3 | 21.4 | |
| International normalized ratio | 1.27 (0.5-1.5) | BE | -2.9 | |
| Thyroid stimulating hormone | 1.4 (0.465-4.68) | Lactate | 1.5 | |
| C-reactive protein | 38.9 (< 1) | Urinalysis | Proteins | 100 |
| Troponin #1 | 0.041 (upper limit 0.034) | Leukocytes | 15 × cap | |
| Troponin #2 | 0.059 (upper limit 0.034) | Erythrocytes | 4-6 × cap | |
| Sodium | 136 (137-145) | Bacteria | Low amount | |
| Potassium | 4.0 (3.5-5.1) | Aspartate aminotransferase | 31.6 (17-59) | |
| Magnesium | 2.0 (1.6-2.3) | Alanine aminotransferase | 19 (< 50) | |
Electrocardiogram on admission: arrhythmic rhythm, variable RR interval, normal axis, heart rate 110 bpm, absent P wave, baseline variation.
Chest radiography on admission: no pathological findings (Figure 2).
A de novo initial diagnosis of complicated UTI and paroxysmal AF was made with CHA2DS2VASc 1 point and HAS-BLED 1 point. Empirical antibiotic management was initiated according to institutional guidelines with aztreonam and blood cultures, urine culture, renal ultrasound, Holter, and transesophageal echocardiography were requested to define possible cardioversion and anticoagulation.
Transthoracic and transesophageal echocardiography
Findings: Moderate dilation of the left atrium with a moderate increase in left atrial volume. Small left atrial appendage, without thrombi, with average Doppler velocities. A membrane’s presence in the left atrium divides the cavity into two chambers, one containing the left atrial appendage and continuing towards the left ventricle and another chamber containing the pulmonary veins. This membrane does not present obstruction to flow due to two fenestrations that communicate in both chambers. Cor triatriatum sinistrum type 2. The other cardiac structures were typical. See supplementary data for video material and their explanations (Figure 3).

Figure 3: Transthoracic and transesophageal echocardiography. The Arrow points to the membrane that divides the left atrium. A) Transthoracic echocardiography, parasternal long axis showing membrane in the left atrium. B) Atrial-focused Transthoracic echocardiography. C-E) Transesophageal image, where the four cardiac cavities are appreciated, in the left atrium, the presence of a membrane that divides is observed. F) Three-dimensional image, showing the membrane in the left atrium.LA = left atrium, LV = left ventricle, RA = right atrium, RV = right ventricle.
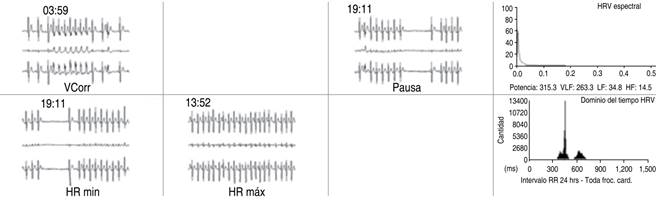
24-hour Holter EKG: baseline sinus rhythm, normal PR interval, QRS complex, and QTc interval. It alternates with multiple episodes of AF with a rapid ventricular response, with pauses after AF cessation. Some episodes of AF with aberration (Ashman phenomenon). Some suggestive episodes of atrial flutter. A mild increase in supraventricular and ventricular automatism (Figure 4).
Report of renal ultrasound finding a prostate enlargement (vol. 95.8 cm3) and a positive blood culture for E. coli ESBL +. Antibiotic management was adjusted according to the antibiogram. The case is evaluated in conjunction with electrophysiology, considering anticoagulation due to cardiac anomaly. Subsequently, the patient resolved bacteremia associated with the UTI and was discharged with a strategy of rate control and anticoagulation with DOAC (direct oral anticoagulant) and outpatient control with Cardiology and Urology.
Discussion
Cor triatriatum (CT) is a rare congenital heart defect whose incidence is estimated to correspond to 0.1% of all congenital heart disease.5 The sinistrum variant is the most frequent and has a more significant association with AF and cerebrovascular attack than CTD.6 The communication between the separate atrial cavities can be wide, small, or null, depending on the fenestrations’ size, presenting as mitral stenosis, generating increased pressure and pulmonary vascular resistance, giving rise to pulmonary arterial hypertension.8 In the literature, symptoms have been attributed to CT only if the transmembrane pressure is > 10 mmHg, and these consist of fatigue, dyspnea, chest pain, palpitations, syncope, exercise intolerance, or even arrhythmias.9 As a diagnostic method, color Doppler echocardiography is the non-invasive modality of choice to identify CT and its hemodynamic impact and recognize associated congenital anomalies and the transmembrane gradient.10 Surgical treatment should be considered when the hemodynamic behavior of the membrane resembles mitral stenosis.11 On the other hand, an incidence of 14.65 vs 12.5% of AF in CTS and CTD, respectively, has been reported in the literature.6 This association is due to the distortion of the architecture and derangement of the muscle fibers in the left atrium, producing remodeling, mechanical and electrical alterations, which ultimately predispose to arrhythmias and thrombus formation.12 The three pillars of AF management are rate control, rhythm control, and anticoagulation. The latter is defined according to the CHA2DS2VASc risk score indicating anticoagulation with 2 points in men and 3 points in women. There has been frequent discussion about whether patients with 1-point CHA2DS2VASc in men and 2-point in women benefit from anticoagulation. Recently, the European Society of Cardiology (ESC) established a reclassification of the risk of cerebrovascular attack in patients with CHA2DS2VASc of 1 point in men and 2 points in women, adding other risk factors such as > 65 years, type 2 diabetes mellitus, persistent or permanent AF, BMI > 30, proteinuria (> 150 mg/24 h), eTFG 1400, positive I/T troponins, enlarged left atrium (volume > 73 mL or diameter > 4.7 cm) and atrial appendage velocity.13
In our patient, CTS’s diagnosis was made late in the seventh decade of life and incidentally due to its little or no hemodynamic impact. The symptoms are not attributed to this cardiac anomaly but its frequent association with arrhythmias such as AF. During the approach to managing his de novo AF to define his criteria for cardioversion, a transesophageal echocardiogram was performed in which CTS was detected. The membrane did not present obstruction flow due to two fenestrations that communicate both chambers, classified as CTS type I. The CHA2DS2VASc risk score was performed, obtaining 1 point indicating no need for anticoagulation due to its low risk of present cerebrovascular events. However, since cerebrovascular attack incidence in patients with AF and CTS/CTD is close to 6.5%, in terms of CHA2DS2VASc, it is equivalent to a score of 4-5 points, so it is decided to anticoagulant with DOAC. There are 13 case reports in the literature in which CTS is associated with cerebrovascular events of cardioembolic origin and evidence of blood stasis or thrombus.12 However, there is still uncertainty about the indication for anticoagulation in this context, given that this cardiac malformation is not frequently considered among the risk factors, which leads to therapeutic dilemmas.
Conclusions
Cor triatriatum is a rare but increasingly recognized congenital cardiac anomaly. Management of this condition depends on the degree of obstruction between the LA chambers seen mainly on Doppler echocardiography. Physiological alterations are like those that occur in mitral stenosis, with alterations in atrial size, volume, and the presence of AF. Current tools or scales for anticoagulation do not consider these types of structural cardiac malformations, as presented in this case.











 nova página do texto(beta)
nova página do texto(beta)