Introduction
Hypertrophic cardiomyopathy (HCM) is the most common inhered cardiac disease. Echocardiography-based epidemiologic studies have shown a prevalence of 1:500 in the general population, but HCM is often underrecognized, especially in women.1 Maron et al. estimated that only 10% of cases are clinically identified (6% symptomatic and 4% asymptomatic), while the other 90% are unidentified.2
Although this disease occurs worldwide, its phenotypic expression and genetic substrate do not fluctuate significantly between populations. HCM is inhered by an autosomal dominant pattern or caused by de novo mutations in 11 or more of the cardiac sarcomere protein genes, with more than 2,000 mutations identified to date. However, the genotype-phenotype correlation has shown variable expressivity and age-related penetrance.1,3,4 It has also been proposed that genetic modifying factors can compensate or aggravate a causal mutation.5
The most common clinical findings in HCM are chest pain, palpitations, congestive heart failure, and sudden cardiac death (SCD). Although clinical manifestations have been most commonly reported between the third and fifth decades of life, significant clinical symptoms in some individuals can appear beyond the seventh decade of life, but this is less well appreciated.6-8
Elderly patients with HCM are more likely to present with congestive heart failure symptoms that are often attributed to more common clinical entities, such as myocardial ischemia, hypertensive heart disease, and aortic stenosis.6 We present a case of late-onset hypertrophic cardiomyopathy referred to our tertiary care center.
Case presentation
An 80-year-old female was sent from a regional hospital’s emergency room to our tertiary referral center with the diagnosis of non-ST-elevation myocardial infarction (NSTEMI). Her past medical history showed insulin-dependent diabetes mellitus and essential hypertension since 30 years ago; currently, under pharmacological control with telmisartan/hydrochlorothiazide (40/12.5 mg), she had no dyslipidemia or smoking history. Her gynecologic history showed G8 P8 and menopause at the age of 50. She had also been studied for a rheumatic valvular disease 15 years ago and had a coronary angiography ten years ago with no significant coronary artery disease. Besides that, she described a persistent atypical chest pain, for which an echocardiogram and a magnetic resonance were performed ten years ago, showing asymmetric septal hypertrophy. However, the intervention was not carried out, attending multiple dyspnea episodes, dizziness, lipothymia, and hypertensive urgencies since then. Nevertheless, she denied a family history of sudden death or cardiomyopathies.
Her current condition began three days before hospital admission with disorientation, dizziness, a blood pressure of 165/70 mmHg, and hyperglycemia, for which she was hospitalized in a regional care center with the diagnosis of NSTEMI. After she was sent to our center, an electrocardiogram was taken, showing a sinus rhythm, incomplete left bundle branch block, inverted T-waves in V4-V6, and no ST-segment elevation. On physical examination, she was aware, oriented, with obesity (BMI of 31.6) and a positive Frank’s sign. To auscultation, an expulsive aortic murmur (III/VI) and a mitral regurgitation murmur (III/VI) were evident. She denied chest pain and dizziness.
Cardiac troponin I (cTpI), creatinine phosphokinase (CPK), and B-type natriuretic peptide (BNP) were ordered. Her levels were: cTpI 1.39 ng/mL, CPK 27 IU/L, and BNP 1200 pg/mL.
A new invasive coronary angiography showed epicardial arteries with no significant lesions (TIMI 3 flow grade). The left basal catheterization showed an aortic pressure of 160/65 mmHg, left ventricular pressure of 280/20 mmHg, and a resting gradient of 120 mmHg. A post-extrasystolic gradient of 240 mmHg was identified (Figures 1A and 1B), described as the Brockenbrough-Braunwald-Morrow sign, demonstrating the presence of left ventricular outflow tract (LVOT) obstruction.9

Figure 1: A) Post-extrasystolic boosting effect, also known as the Brockenbrough-Braunwald-Morrow sign. Note the presence of a gradient of 240 mmHg between the left ventricular (LV) and aortic pressures (Ao) after the premature ventricular contraction (PVC). B) Transaortic gradient observed in each pacemaker stimulation (arrows).
A transthoracic (TTE) and a transesophageal echocardiogram (TEE) were requested to determine the gradient site and assess the possibility of alcohol septal ablation. The TTE showed a predominantly anterior septal hypertrophy, with a maximum thickness of 27 mm; significant left atrial dilation, with an increased volume of 56 ml (Figure 2A); mitral valve sclerosis with calcification spots and mild-moderate mitral regurgitation. The TEE showed generalized low-grade hypokinesia, left ventricular ejection fraction (LVEF) of 64%, and an intraventricular gradient at rest greater than 168 mmHg, with an anterior systolic movement of the anterior mitral leaflet (Figure 2B).

Figure 2: A) Two-dimensional echocardiogram image. Four-chamber view showing asymmetric septal hypertrophy and biauricular dilatation. B) Transesophagic echocardiogram showing an intraventricular gradient pressure at the rest of 168 mmHg (white arrow).
Due to the LVOT obstruction, the persistent symptoms, and the comorbidities previously mentioned, together, the hemodynamic, echocardiography, and cardiology teams decided to schedule the alcohol septal ablation. Through a trans-jugular approach, a temporary pacemaker was placed in the right ventricle. A guide was advanced through the left coronary artery to the first septal artery using a bilateral radial approach (Figure 3A), where an OTW balloon catheter was dilated (2.0 × 1.5 mm) until the complete occlusion was verified. With echocardiographic support, the right anatomical site was also confirmed. Initially, 7 ml of 70% ethanol was slowly injected, but only a partial occlusion was observed (Figure 3B); thus, an additional 3 mL injection was added, observing a complete embolization (Figure 3C). No complications in the anterior descending coronary artery or electrical abnormalities were detected, but it was decided to leave the pacemaker implanted on ventricular demand pacing. The post-extrasystolic intraventricular gradient was measured again, and a minimum residual gradient was recorded (aortic pressure of 160/55 mmHg, left ventricular pressure of 190/40 mmHg, and a peak gradient of 30 mmHg), while the resting gradient was absent (Figure 4). No periprocedural complications were presented.
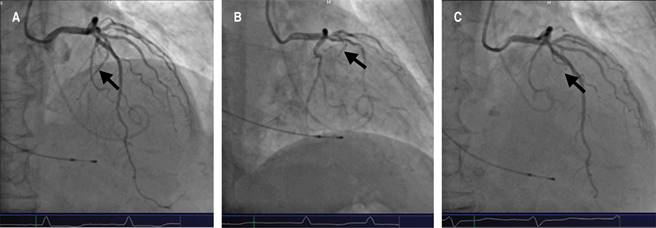
Figure 3: A) Coronary angiography, where the first septal artery is observed as a branch of the anterior descending coronary artery (Arrow). B) The first septal artery is occluded with a balloon, and the alcohol is injected. C) The absence of blood flow on the first septal artery is observed after the alcohol septal ablation.

Figure 4: The absence of a significant transaortic gradient was observed after alcohol septal ablation.
She was admitted to the coronary intensive care unit for close monitoring of possible complications. She remained asymptomatic on physical examination, and her mesotelesystolic aortic murmur decreased in intensity (I/VI). A new echocardiogram was performed, showing an LVOT mean gradient of 57 mmHg and akinesia of the basal anteroseptal wall. After 72 hours, she was discharged from the hospital without any cardiovascular or metabolic complications.
Discussion
HCM is defined as a nondilated left ventricular hypertrophy (LVH), identified through echocardiography or magnetic resonance imaging, which occurs in the absence of another cardiac, metabolic, or systemic disease. The typical pattern of HCM is the asymmetric septal hypertrophy, but other forms can occur.1,10 HCM is predominantly an obstructive heart disease,11 usually produced by mitral-valve systolic anterior motion and septal contact due to flow drag, showing mitral regurgitation.1
Previously, HCM had been reserved as a relevant clinical entity in the young population because of its strong association with a higher risk of SCD in adolescents and young adults due to coronary microvascular dysfunction and ischemia.1 Now, it is well known that the clinical course of HCM covers a broad spectrum of manifestations, where many patients will remain asymptomatic with an average life expectancy, but others will progress along with the disease’s natural history and its complications.8,12,13
Although SCD is one of the most feared complications, HCM patients who survive beyond the age of 60, paradoxically, seem to be protected against SCD (similar rates compared with the general population).1,6 These patients can have more than one conventional clinical marker associated with increased risk for premature SCD, but they do not have the same prognostic significance. Therefore, the recommendation for primary prevention with implantable defibrillators may be unnecessary in patients who have survived for many decades.8 Other relevant features in elderly HCM patients are the risk of atrial fibrillation and consequent embolic stroke.7
According to various genetic-based studies, mutations in the cardiac myosin-binding protein C (MYBPC3) gene are the most common cause of delayed hypertrophy expression (late-onset HCM).7 It has a more benign prognosis than mutations in other sarcomeric protein genes, such as the β-myosin heavy chain (MYH7) and troponin T (TNNT2).4,5 Family screening must be performed with a phenotypic or genotypic strategy, depending on the genetic test availability, although the yield of genetic testing is lower for elderly patients without a family history of HCM.14
Imaging findings variate from young patients to elderly ones. Wall thickness is more significant in the young population than elderly patients with less severe hypertrophy, related to a better prognosis. Older patients also have shown less diffuse myocardial involvement, often confined to the ventricular septum. Key features that distinguish HCM from hypertensive heart disease are the asymmetric distribution of the hypertrophy and left ventricular outflow tract (LVOT) obstruction.6,15
Management options in symptomatic patients with hypertrophic obstructive cardiomyopathy (HOCM) are β-blockers, non-dihydropyridine calcium channel blockers, and disopyramide, alone or in combination. Hypertension is frequent as a comorbid condition in these types of patients, and many antihypertensive drugs are relatively contraindicated in HOCM because of the risk of decreased cardiac output, such as diuretics and vasodilators.16
In addition to medical therapy, HOCM patients can be approached by surgical myectomy or alcohol septal ablation to reduce the LVOT gradient. Alcohol septal ablation has gained popularity since it uses a transcoronary administration of ethanol via a percutaneous approach to induce a localized infarction of the basal septum, where the anterior mitral valve leaflet is located.17 The American College of Cardiology Foundation/American Heart Association guidelines on HCM state that alcohol septal ablation should be reserved for elderly patients and patients with severe comorbidities.17 A recent meta-analysis showed that alcohol septal ablation is associated with lower periprocedural complications but higher re-interventions and pacemaker implantation rates due to atrioventricular block.18 The 5- and 10-year survival rates following alcohol septal ablation in patients older than 55 years are 93% and 82%, respectively, and the risk of adverse arrhythmic events is low (1.4% per year), comparable to age-matched non-obstructive HCM patients.19
The life expectancy of late-onset HCM patients beyond the seventh decade of life is likely to be more influenced by their comorbidities than HCM itself. In a study with 428 patients between the ages of 60 to 91 years at study entry, survival at 5 and 10 years (accounting for all-cause mortality) was 77% and 54%, respectively. Mortality events related to HCM were 0.64% per year due to atrial fibrillation-related embolic stroke, arrhythmic sudden death events, progressive heart failure, postoperative complications, and heart transplantation for end-stage disease.20
For the reasons mentioned above, the management of elderly HCM patients must be individually evaluated by a multidisciplinary team, taking into account the clinical presentation, comorbidities, mitral valve anatomy, coronary anatomy, septal thickness, and the hospital’s expertise for each procedure.19
Conclusions
Late-onset HCM usually comprises a more benign spectrum of the disease, but severe cases may occur. Despite being uncommon in the elderly, it should be included in the differential diagnosis of congestive heart failure and myocardial ischemia. A multidisciplinary team must individually assess the clinical characteristics to decide the best therapeutic approach for the patient.











 nueva página del texto (beta)
nueva página del texto (beta)


