Introduction
Historically, spontaneous coronary artery dissection (SCAD) was considered a rare cause of acute coronary syndrome (ACS) and sudden cardiac death, with an incidence of 0.1% to 4%.1-4 However, in the last decade, this entity has lost the designation of «rare» condition, being recognized as a cause of myocardial infarction and cardiac death, especially in young women.5,6 Myocardial infarction as a result of SCAD has been documented in adolescence up to the ninth decade of life and, although its physiopathology is still a subject of controversy, there is a combination of predisposing factors that can influence and increase the susceptibility to develop this pathology, including sex, hormonal fluctuations, collagen diseases and physical or emotional precipitants.6,7 Women account for more than 90% of SCAD cases, so sex hormones have been implicated in its development.4,5,7,8 However, there are reports of cases in women with SCAD, without hormonal influences.3,9 Other conditions such as basal artery disease are frequent, with fibromuscular dysplasia being the associated pathology in > 70%; and other connective tissue diseases such as Marfan, Loeys-Dietz, and Ehlers-Danlos in a smaller proportion, of 5%.7,10
SCAD is an acute coronary event that is presented by the development of a hematoma within the medial tunic, producing a separation of the arterial intima and the consequent compression of the true lumen.7 This process can be carried out by two different mechanisms: 1) spontaneous disruption of the intima, creating an entry point for the accumulation of an intramural hematoma in the false lumen, leading to the separation of the arterial wall, and 2) hemorrhage from the vasa vasorum into the arterial wall, resulting in an intramural hematoma, with expansion and increased pressure in the false lumen and external pressure in the true coronary lumen.5,11
Coronary angiography represents the main diagnostic method for SCAD, with the indication to resort to intracoronary imaging methods only in cases where there is doubt about the diagnosis or where percutaneous coronary intervention (PCI) is required6,7 PCI has 57% of successful procedures,6,12 being associated with high rates of complications. Typically, conservative therapy is the most accepted because of its high rate of complete resolution over time2,6 with values of up to 86% spontaneous healing in control angiography.13
Case report
28 year old male patient, of Chinese descent, cook, active smoker from 20 to 28 years old, five cigarettes a day with a tobacco index of two, with no other cardiovascular risk factors, refers not to consume medicines, drug addiction not reported as cocaine or intravenous drugs, no blood or urinary toxicological tests were performed. He has a family history of a mother who died at age 50 from heart disease and a sister with a current diagnosis of heart disease (both with unspecified heart disease). He started the symptoms suddenly while working, presenting dyspnea and thoracic pain of oppressive characteristics, 10/10 intensity, diaphoresis than lasted for more than an hour. He had no previous history of angina. He arrives at the emergency department where is observed, diaphoresis, facies of distress, and hemodynamic instability (BP 80/50 mmHg, HR 112 bpm, RR 15 bpm and SaO2 90% ambient air). On physical examination there was no murmurs were found, and no third or fourth sounds, were noticed. No other signs of heart failure were detected. It is worth mentioning that in the context of emergency care for acute coronary ischemic syndrome, physical examination revealed only a fair complexion and short stature (158 cm) for his age and gender. No long arms, legs, fingers and toes, flexible joints or very elastic skin were documented, which would point to a group of hereditary connective tissue disorders associated with Marfan or Ehlers-Danlos syndrome.
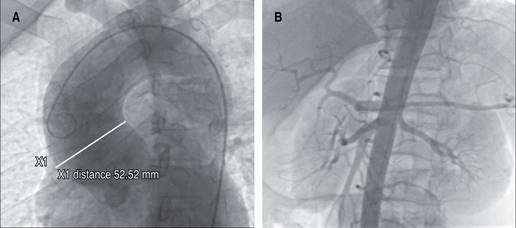
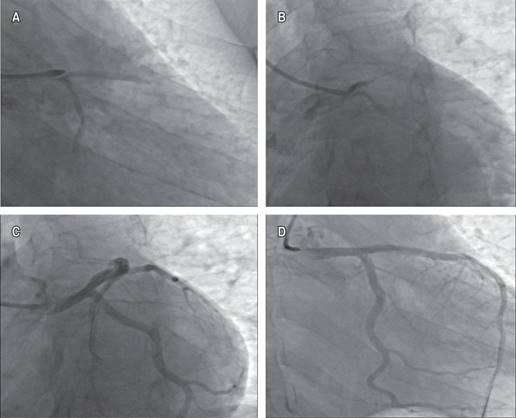
The initial electrocardiogram showed sinus rhythm with ST segment elevation of 2 mm in aVR and V1, and ST segment depression in DII, DII aVF, and V3 to V6 (Figure 1). A clear evidence of elevated cardiac enzymatic markers was documented, with troponin T levels > 30 ng/mL. Coronary angiography was performed, finding significant dilatation of the ascending aorta (Figure 2). The urgency of the case, the evidence of SCAD and the patient’s clinical condition prevented the implementation of intracoronary imaging. Antegrade flow TIMI 2 towards circumflex and anterior descending was shown (Figure 3). It was decided to implant a direct drug-eluting stent 4.5 × 24 mm, obtaining a TIMI 3 antegrade flow and pain reduction. In stable condition was admitted to the intensive care unit. However, during his 24-hour surveillance he presented acute heart failure data and tissue hypoperfusion: dyspnea, tachycardia, pulmonary congestion, and SaO2 80%, requiring mechanical ventilation, and the use of inotropes and vasopressors. The control electrocardiogram (Figure 1) showed bursts of sustained ventricular tachycardia treated with amiodarone infusion. Transthoracic echocardiogram documented systolic dysfunction with left ventricular ejection percentage of 40%, severe anterior hypokinesia, and no evidence of mechanical complications. Hours later (24 hours after the coronary intervention), he presented sustained ventricular tachycardia and later asystole without response to cardiopulmonary resuscitation maneuvers, which could suggest a total occlusion of the anterior descending artery, related to a possible propagation of a intramural hematoma distal to the lesion. Unfortunately, the autopsy was not carried out due to the refusal of the relatives.
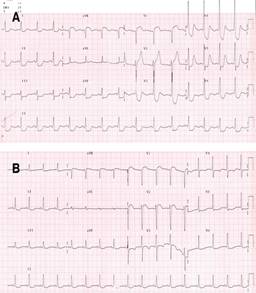
Figure 1: A) Initial 12-lead electrocardiogram. B) Control electrocardiogram, post percutaneous coronary intervention (PCI).
Discussion
SCAD is an increasingly recognized cause of acute coronary syndromes and cardiovascular death. In recent years, the impact of an early invasive approach with coronary angiography on patients with ACS, and the more recent adoption and accessibility of intracoronary imaging methods as a diagnostic tool such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT),11 has led physicians to pay more attention to reassessing this entity in greater depth.5,12
Although the mechanism by which the dissection develops is known, different clinical characteristics and predisposing factors influence its process. These factors, the clinical presentation and natural history of the disease, are documented mainly in women, since more than 90% prevail in them, while cases of SCAD in men have been little described in the literature.8,13,14 The risk factors most frequently associated in men (in addition to those already commonly described in both sexes) are isometric stress, active smoking and drug use,3,14 however, these are a minority. SCAD is associated with predisposing arteries, with connective tissue diseases, autoimmune or inflammatory diseases.11 Fibromuscular dysplasia shows the highest correlation with SCAD, > 70%.5,6,9 Any artery can be affected in SCAD, however, it has been found that the anterior descending artery is the most affected (middle to distal) and its branches, producing 45 to 60% of the cases; followed by the circumflex artery and its branches with 15 to 45%; and a lower incidence, when dealing with the left coronary trunk, 0-4%.5,7,12
Spontaneous dissection of the left main coronary artery is extremely rare and, regarding the present clinical case, a strong association is suggested with the angiographic finding of dilatation and aneurysmal disease of the ascending aorta. There are various anomalies whose main pathophysiological finding is mainly limited to disproportionate aortic dilation, within which systemic inflammatory diseases such as Takayasu disease can be included, which although it is rare, with a reported incidence of 2-3/1,000,000 per year,15,16 being most common in young adults between the second and third decade of life, especially Asian women.2,3 This motivated us to consider the patient’s family history.
One of the main genetic contribution to the disease pathogenesis is supported mainly by its association with the HLA (Human Leukocyte Antigen) complex, which associations are varied according to the patient ethnic background. The strongest association has been established with HLA-B52 in Japanese population, as well as associations with HLA-B5 described in patients with Asian and Mexican Mestizo background and certain polymorphisms HLA-B in Chinese population. Although the mechanism described by which distal ischemia usually develops in Takayasu disease is due to occlusion and stenosis of the vessels, a mechanism of dissection of the main branches; such as the left coronary artery in the present case, is not ruled out due to the weakening and destruction of its muscular and elastic layers.10,14,15
Cystic media degeneration is a histopathological finding characterized by an accumulation of basophilic ground substance in the media, degenerative disruptions of collagen, elastin and smooth muscles may result in weakening of the arterial wall.17 This phenomenon might be the underlying cause of left main coronary artery dissections due to its uncommon location; even though it can be found in many other pathologies, it is mainly associated with connective tissue disorders such as Marfan syndrome.18 However, no phenotypic features of Marfan syndrome were observed in the patient, thus an association cannot be made.
The decision to revascularize the patient though PCI was based upon the compromised anatomy, clinical presentation; left main involvement and hemodynamic instability.7,11,19 There were many modulating factors and triggers that, in the context of a previously vulnerable myocardium, could have generated a rapidly growing hematoma that expanded and dissected all the way to de anterior descendant artery which is, in fact, one of the most frequently reported complications of PCI in patients with SCAD8 (Tables 1 y 2),3,6 leading to distal occlusion, ischemia and electrical instability that finally led to a fatal outcome. This rapid sequence of events limited the definitive diagnosis of his underlying pathology that led to SCAD, and protocolize and stratify his treatment.
Table 1: Comparison of outcomes following SCAD from 5 published series.
| Study lead author | Death (in hospital) | Death post discharge (median follow-up in months) | Recurrent AMI post discharge (median follow-up in months) | Recurrent de novo SCAD (median follow-up in months) |
|---|---|---|---|---|
| Tweet | 1/189 | 3/189 (27) | 37/189 (27) | 29/189 (27) |
| Lettieri | 3/134 | 4/127 (22) | 2/127 (22) | 6/127 (22) |
| Rogowski | 1/64 | 0/64 (54) | 3/64 (54) | 3/64 (54) |
| Nakashima | 0/63 | 1/63 (34) | 18/63 (34) | 7/63* (34) |
| Saw | 0/327 | 4/327 (37) | 55/327 (37) | 34/327 (37) |
AMI = acute myocardial infarction; SCAD = spontaneous coronary artery dissection.
Source: Adlam D et al.6
* Recurrent events in this series reported as events beyond 30-days after index SCAD event.
Table 2: In-hospital and 30-days cardiovascular events in 750 patients.
| n (%) | |
|---|---|
| Overall in-hospital MAE | 66 (8.8) |
| 95% CI 6.9-11.1 | |
| Death | 1 (0.1) |
| Recurrent MI | 30 (4.0) |
| Extension of SCAD segment | 15 (50.0) |
| Iatrogenic dissection | 9 (30.0) |
| Other | 6 (20.0) |
| Severe ventricular arrhythmia | 29 (3.9) |
| Requiring ICD | 6 (0.8) |
| Haemodynamic instability | 15 (2.0) |
MAE = major adverse events; MI = myocardial infarction; SCAD = spontaneous coronary artery dissection; ICD = implantable cardioverter defibrillator.
Source: Saw J et al.3
Conclusions
Spontaneous dissection of the left main coronary artery is an uncommon condition, often with fatal outcomes. Although there are case reports in the literature, no precise associations have been described with diseases or structural alterations of the ascending aorta, which are suspected to have been involved in this patient, whose acute clinical condition allowed timely percutaneous coronary intervention, with a successful angiographic result. Post-procedure complications prevented identification of the cause of cardiac arrest by invasive and non-invasive tests, making it impossible to perform a new diagnostic-therapeutic coronary angiography. For future cases, the use of intracoronary images will be necessary to measure the size of the stent according to the longitudinal extension of the false lumen, to minimize complications such as the spread of the intramural hematoma. As well as the intracoronary images after the intervention, to evaluate the adequate stent expansion. Finally, the result urges us to include for early scrutiny, the scarcely documented modalities associated with this entity, to generate awareness and suspicion of a preventive nature in the treating physician when faced with probable cases of SCAD and not to wait for its result as a finding in a necropsy.











 nueva página del texto (beta)
nueva página del texto (beta)