Introduction
PCSK9 (proprotein convert subtilisin/kexin type 9) is a plasma enzyme that binds the low-density lipoprotein receptor on hepatocytes’ surface, thus promoting their metabolism and subsequent degradation in lysosomes.1 The use of monoclonal antibodies against PCSK9 increases the half-life of the low-density lipoprotein receptor, leading to a reduction in the plasma concentration of low-density lipoprotein cholesterol (LDL-C) and a lower long term cardiovascular risk.2,3
Evolocumab was the first monoclonal antibody against PCSK9 to be authorized as a lipid-lowering drug. It is indicated for adult patients with familial heterozygous hypercholesterolemia, familial homozygous hypercholesterolemia, non-familial hypercholesterolemia and primary mixed dyslipidemia. Additionally, it has been used for treatment in patients with myocardial infarction that does not respond to statin therapy.2,3
In the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial, treatment with evolocumab for 48 weeks reduced LDL-C levels from an average of 92 to 30 mg/dL, with an average percentage reduction of 59% (95% CI, 58-60). Also, evolocumab reduced the risk of cardiovascular events, non-HDL-C and apolipoprotein B levels.4
In the YUKAWA-2 (Study of LDL-Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients with Advanced Cardiovascular Risk) trial, performed in Japanese individuals at high risk of cardiovascular disease, evolocumab in combination with atorvastatin was found to reduce LDL-C concentration by 60-70%. The increased reduction in LDL-C concentration of this trial, compared to the FOURIER4 and LAPLACE-2 (LDL-C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy)5 trials, is due to intrinsic differences in the Japanese population at high cardiovascular risk and not to complementary treatments or the baseline levels of LDL-C or PCSK9.6 Other phase III clinical trials have been consistent in showing a reduction of LDL-C with evolocumab treatment.7,8
Recently, the European Society of Cardiology (ESC) has set new targets in managing of patients at very high cardiovascular risk. A target concentration of LDL-C ≤ of 55 mg/dL with a reduction of more than 50% has been established.9 These targets are more ambitious than recommendations outlined in 2016.10 Therefore, therapies that combine a PCSK9 inhibitor and statins are a treatment option to achieve the new target LDL-C concentrations in patients at very high cardiovascular risk.
Despite the benefits of evolocumab shown in previous studies, this drug’s general use is uncommon due to its relatively high cost compared to statins.11 Thus, a cost-benefit assessment of treatment with evolocumab should first be considered.12 As shown in previous studies, the benefit of this treatment depends on the intrinsic characteristics of the population to be treated.6 Therefore, it is necessary to evaluate the efficacy and safety of evolocumab treatment and to establish whether the new objectives set by the ESC for patients at very high cardiovascular risk can be achieved. In the present study, we evaluated levels of LDL-C, HDL-C, total cholesterol, and triglycerides, before and after treatment with evolocumab in combination with atorvastatin.
Objective: To evaluate the efficacy of evolocumab to achieve the target LDL-C levels in patients with ischemic heart disease at very high cardiovascular risk.
Material and methods
A pre-experimental study was conducted and approved by the Committee of Health Research and Ethics Research with registration number 043/18. The procedures followed were per relevant clinical research ethics committee regulations and with those of the Code of Ethics of the World Medical Association (declaration of Helsinki). Twenty patients with a diagnosis of ischemic heart disease diagnosed by coronary angiography were included in the study. The patients were treated at the Cardiology Department of the Instituto de Seguridad Social del Estado de México y Municipios. The inclusion criteria for this study were males and females 18 years or older, diagnosis of ischemic cardiopathy, and failure to reach a concentration ≤ 55 mg/dL of LDL-C after treatment with statins at the maximum dosage for three months or longer. All patients were classified to be at very high cardiovascular risk based on ESC criteria, by a cardiologist.9
Patients were treated with evolocumab (140 mg, subcutaneous dose) every two weeks. Additionally, 80 mg of atorvastatin was administered every 24 hours as an adjunct therapy, except for one patient who was only treated with evolocumab due to an intolerance to atorvastatin. Both treatments were administered for 24 weeks. At the end of this period, total cholesterol, triglycerides, LDL-C, and HDL-C concentrations were determined by commercial methods (Beckman). Neurocognitive impairment was determined by applying the mini-mental state examination (MMSE) test, adapted to the Mexican population,13 before and after treatment. The MMSE test addresses the following five cognitive domains: temporospatial orientation, deferred memory, attention and computation, language, and visuoconstructive drawing ability.14
Results
The patients treated included six women and 14 men of mixed race, with an average age of 58 ± 9.9 years. All patients presented previous cardiovascular complications, 20 patients had acute coronary ischemic syndrome, a patient had ventricular tachycardia, and another had a third-degree atrioventricular block. All patients were classified at very high risk according to ESC criteria.9 Intolerance to statins was considered to be any adverse event secondary to the drug administration that led to the impossibility of its use. During the study, one patient presented intolerance to atorvastatin by referring to muscle pain, which led to atorvastatin’s withdrawal. No biochemical or functional alteration was observed in the laboratory exams or cabinet studies. The clinical data of the patients are shown in Table 1.
Table 1: Clinical characteristics of the patients studied.
| Patient characteristics | Number of patients |
|---|---|
| Total patients | 20 |
| Age | 58 ± 9.9 years |
| Females | 6 |
| Diabetes mellitus 2 | 10 |
| Systemic arterial hypertension | 18 |
| Smoking | 11 |
| Dyslipidemia | 20 |
| Ischemic heart disease | 20 |
| Previous cardiovascular complications | 20 |
| Pre-treatment | |
| Statin | 17 |
| Fibrates | 4 |
| Ezetimibe | 4 |
| Statin intolerance | 1 |
The average LDL-C concentration before treatment with evolocumab was 138.3 ± 15 mg/dL. After 24 weeks of treatment, a significant reduction was found (p < 0.001) in LDL-C concentration with a final average of 62.2 ± 7.5 mg/dL (Figure 1A). The average percentage reduction in LDL-C was 55% (95% CI, 45 to 65). Conversely, the average HDL-C concentration before treatment (45.8 ± 2.8 mg/dL) and after treatment (47 ± 2.3 mg/dL) was not significantly different (Figure 1B).
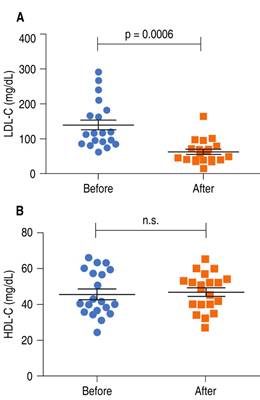
Figure 1: Treatment with evolocumab decreases the concentration of LDL-C but not HDL-C in plasma. The graph shows the concentration in mg/dL of LDL-C (A) and HDL-C (B) of 20 patients before and after treatment with evolocumab. The horizontal lines indicate the mean and standard error. The data were compared with the Wilcoxon matched-pairs test.
Treatment with evolocumab reduced plasma cholesterol concentration (Figure 2A) but not triglyceride concentration (Figure 2B). The average total cholesterol concentration before treatment (203.0 ± 15.2 mg/dL) and after treatment (147.4 ± 12.1 mg/dL) was significantly different (p < 0.001). Triglyceride concentration was 224.2 ± 41.7 mg/dL before treatment and 206.1 ± 23.4 mg/dL after treatment. One patient had severe heel pain after the administration of the antibody, although not attributed as a side effect. No neurocognitive adverse events were observed either.

Figure 2: Treatment with evolocumab reduces the concentration of total cholesterol in plasma but not triglyceride concentration. The graph shows the concentration in mg/dL of total cholesterol (A) and triglycerides (B) of 20 patients before and after treatment with evolocumab. The horizontal lines indicate the mean and standard error. Total cholesterol and triglyceride concentrations were compared with the Wilcoxon matched-pairs test.
Discussion
In our study, patients at very high cardiovascular risk were treated with 140 mg of evolocumab every two weeks, combined with atorvastatin. Clinical trials of evolocumab, alone or in combination with statins, have shown that treatment with 140 mg every two weeks or with 420 mg once a month are of equal efficacy.4,6,8
Patients had previously been treated with ezetimibe in combination with high-intensity statins, yet LDL-C levels were not achieved. For this reason, it was decided to add evolocumab to the therapy, in combination with a high-intensity statin. Dual therapy was maintained in our study, but in a Mexican population, as in the YUKAWA-2 and LAPLACE-2 studies, since in these studies a significant reduction of LDL-C was achieved and the target levels of LDL-C were reached.6,15 Therefore, the objective of the present study was to evaluate if the target level of LDL-C could be achieved in the Mexican population without having to use an additional drug. Using two drugs is advantageous because it allows for greater adherence to treatment. Administration of more than two drugs correlates with poor treatment adherence, a key factor in lipid-lowering therapies.16
The average percentage reduction of LDL-C concentration in this work was 55% (95% CI, 45-65). This percentage of reduction was similar to the one obtained in the FOURIER trial, where an average percentage reduction for LDL-C of 59% (95% CI, 58-60) was obtained.4 The significant variability in our results can be attributed to the sample size. In the YUKAWA-2 trial, a 75.9% reduction in LDL-C concentration was found in Japanese patients at very high cardiovascular risk treated with 140 mg of evolocumab every two weeks combined with 20 mg/day of atorvastatin.6 This percentage reduction was higher than that reported in our study, although the atorvastatin dose in our study was four times higher. This more significant reduction in LDL-C concentration was not due to the differences at the baseline level since the YUKAWA-2 trial baseline was 109 ± 35 mg/dL, and in our study, the baseline was 138 ± 67 mg/dL, which are not statistically different. Although the reduction in LDL-C in our study was not as pronounced as that observed in the YUKAWA-2 trial, 11 of 20 patients managed to achieve an LDL-C concentration ≤ 55 mg/dL, the recommended level by the ESC.
In our work, we did not find an increase in HDL-C concentration after treatment with Evolocumab. However, other studies have reported an increase in HDL-C levels. For example, in the YUKAWA-2 trial, evolocumab increased HDL-C by 10-17%.6,17 A direct mechanism can be attributed to this increase in HDL-C concentration has not been evaluated yet. However, a possible indirect mechanism may act through the decrease in the concentration of LDL-C, which would affect the enzymatic activity of cholesteryl ester transfer protein, an enzyme that transfers cholesterol from HDL to LDL.6
In previous reports, there has been a low rate of complications inherent to treatment with evolocumab. In the FOURIER study, only 0.1% of patients stopped treatment due to reactions at the puncture site.4 In our study, there were no complications related to medications, and all patients finished the entire treatment. No cases of neurocognitive adverse events were reported after 24 weeks of treatment. Long-term studies such as EBBINGHAUS (Evaluating PCSK9 Binding antiBody Influence oN coGnitive HeAlth in High cardiovascUlar Risk Subjects) study did not find differences in cognitive function between patients treated with evolocumab and the placebo group, as well.18
A limitation of the present study is the lack of ezetimibe as a complementary treatment. Possibly, the combined use of the three therapies (evolocumab, atorvastatin, and ezetimibe) will allow a greater number of patients to achieve target C-LDL levels. Although this study has its limitations such as the length of time for treatment and sample size, results showed that treatment with evolocumab combined with atorvastatin reduces LDL-C concentration to the recommended levels. However, not all the patients achieved the LDL-C target level, and given the high cost of the drug, cost-benefit analyses are required to assess therapy with evolocumab.
Conclusions
A 55% reduction in LDL-C concentration was observed in patients treated with evolocumab in combination with atorvastatin. The LDL-C level recommended by the ESC was reached in 11 of the 20 patients treated. Given the high cost of treatment with evolocumab, additional strategies are required to achieve the target LDL-C level in a greater patients population.











 nueva página del texto (beta)
nueva página del texto (beta)


