Introduction
Despite good progress in the management of patients with atrial fibrillation (AF), it remains one of the major causes of stroke, heart failure, sudden death, and cardiovascular morbidity in the world. Furthermore, the number of patients with AF is predicted to rise steeply in the coming years1. Post-operative atrial fibrillation (POAF) is common after both cardiothoracic and non-cardiothoracic surgery. AF has been reported in up to 5-40% of patients in the early post-operative period following coronary artery bypass graft (CABG)2. Patients developing post-operative AF usually do not have a previous arrhythmic history3.
Early risk detection of AF would contribute to the prevention and enable forehand treatment with proper medications. Post-operative AF most frequently occurs on the 2nd or 3rd day after CABG. Seventy percent of patients develop this arrhythmia before the end of post-operative day four4.
Optimal risk assessment needs to be done 24 h before possible fibrillation appearance since prophylactic medication must be administered promptly5.
In recent years, advances in surgery, surgical techniques, cardiopulmonary bypass (CPB), cardioplegic arrest, aortic cross-clamping time, anesthesia, and post-operative care operative have led to declining of post-operative care operative mortality and morbidity. However, the incidence of post-operative atrial fibrillation has not decreased and appeared to be increasing, most likely attributable to the increasing proportions of CABG procedures performed in elderly patients6. This study aimed to detect the coronary angiographic characteristics for the prediction of post-operative atrial fibrillation in patients with ischemic heart disease undergoing coronary artery bypass grafting
Methods
This was a prospective and observational clinical study that was conducted at National Heart Institute and at Cardiac and Thoracic Academy at Ain Shams University from September 2018 to September 2019.
This study included 100 consecutive patients with coronary artery disease (CAD) and sinus rhythm scheduled for coronary artery bypass graft.
The exclusion criteria were:
Rhythm other than sinus.
Impaired left ventricle systolic function (EF < 40%).
Congenital heart diseases.
Associated aortic and mitral valve diseases indicated for concomitant aortic or mitral valve replacement.
Previous cardiac operation. Patients had previous percutaneous coronary intervention (PCI) not excluded.
Data collected are:
Review of medical history
This included demographic data (age, gender, and body mass index), risk factors as smoking, hypertension, diabetes mellitus, and dyslipidemia.
Electrocardiography
Twelve leads resting ECG was done for each patient pre-operative and post-operative.
Pre-operative ECG was done as baseline, confirming the presence of sinus rhythm and for comparing it with post-operative ECG to detect whether the patient developed AF or not. Post-operative ECG was done daily in the intensive care unit and before discharge.
Echocardiography
All patients were examined in the left lateral position with TDI software. All echocardiographic and Doppler data were obtained in digital format and stored for offline analysis.
LA Volumes: LA passive maximal LA volume (V max), measured just before the opening of the mitral valve in end-systole7.
LV volumes and left ventricular ejection fraction:
Global LV function was assessed by measuring LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LVEF from the conventional apical 2-&4-chamber views, using the biplane Simpsons method8.
Results
Regarding the risk factors, there was no significant statistical difference between the two groups except for the age of patients, which was higher in Group I.
Patients demographics
Patients of Group I were significantly older than Group II (mean age of 65 ± 6.25 in Group I versus 54.5 ± 6.7 in Group II, p = 0.001) (Table 1). While the difference between the two groups, regarding risk factors, was insignificant (Table 2).
Table 1 Patients demographic as regard age
| Age | Group 1 (POAF) | Group 2 (NSR) | t-test | |
|---|---|---|---|---|
| t | p - value | |||
| Range | 50 - 75 | 38 - 68 | 6.185 | < 0.001* |
| Mean ±SD | 64.455 ± 6.254 | 54.577 ± 6.710 | ||
Table 2 Patients demographic as regard risk factors
| AF | Chi-Square | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 (POAF) | Group 2 (NSR) | Total | |||||||
| n | % | n | % | n | % | X2 | p-value | ||
| HTN | + ve | 18 | 81.82 | 53 | 67.95 | 71 | 71.00 | 1.603 | 0.205 |
| − ve | 4 | 18.18 | 25 | 32.05 | 29 | 29.00 | |||
| DM | + ve | 16 | 72.73 | 46 | 58.97 | 62 | 62.00 | 1.378 | 0.241 |
| - ve | 6 | 27.27 | 32 | 41.03 | 38 | 38.00 | |||
| Smoking history | + ve | 15 | 68.18 | 46 | 58.97 | 61 | 61.00 | 0.612 | 0.434 |
| - ve | 7 | 31.82 | 32 | 41.03 | 39 | 39.00 | |||
| Dyslipidemia | + ve | 15 | 68.18 | 62 | 79.49 | 77 | 77.00 | 1.238 | 0.266 |
| - ve | 7 | 31.82 | 16 | 20.51 | 23 | 23.00 | |||
Diseased vessels
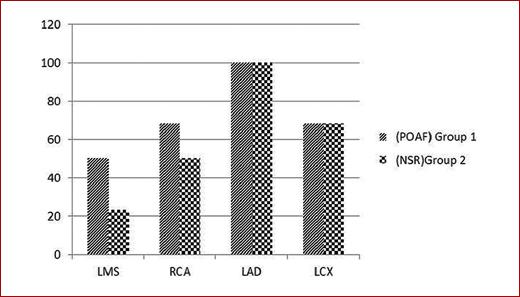
Left main coronary artery involvement was higher in patients who developed post-operative AF. Eleven patients of Group I had lesions in LM (50%) versus 18 patients of Group II (23.07%), p = 0.014 (Table 3), 15 patients had RCA lesions of Group I (68.18%) versus 39 patients of Group II (50%), p = 0.131, and 15 patients had LCX lesions of Group I (68.18%) versus 53 patients of Group II (67.94%), p = 0.983, while all patient who had LAD lesion in both groups (Table 4, Fig. 1).
Table 3 Comparison between the two groups regarding the left main lesion
| Left main | AF | Chi-square | ||||||
|---|---|---|---|---|---|---|---|---|
| Group I (POAF) | Group II (NSR) | Total | ||||||
| n | % | n | % | n | % | X2 | p-value | |
| Positive | 11 | 50.00 | 18 | 23.08 | 29 | 29.00 | 6.041 | 0.014* |
| Negative | 11 | 50.00 | 60 | 76.92 | 71 | 71.00 | ||
| Total | 22 | 100.00 | 78 | 100.00 | 100 | 100.00 | ||
Left main coronary artery involvement was higher in patients who developed post-operative AF. Eleven patients of Group I had lesions in LM (50%) versus 18 patients of Group II (23.07%), p value = 0.014, while there is no significant difference between the two groups regarding other coronaries.
Table 4 Comparison between the two groups regarding LAD, LCX, and RCA lesions
| Group I | Group II | p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| RCA | 15 | 68.18 | 39 | 50.0 | 0.131 |
| LAD | 22 | 100.0 | 78 | 100.0 | NA |
| LCX | 15 | 68.18 | 53 | 67.94 | 0.983 |
Fifteen patients had RCA lesions of Group I (68%) versus 39 patients of Group II (50%), p-value = 0.131, 15 patients had LCX lesions of Group I (68.18%) versus 53 patients of Group II (67.94%), p-value = 0.983. No significant difference between the two groups regarding LCX OR RCA lesions.
There was no significant statistical difference between the two groups regarding the numbers of implanted grafts. One patient (4.54%) of Group I versus 12 patients (15.38 %) of Group II had single-vessel disease, p = 0.072, 12 patients (54.54 %) of Group I versus 40 patients (51.28 %) of Group II had two vessels disease, p = 0.071, and 9 patients (40.90%) of Group I versus 26 patients (33.33%) of Group II has three vessels disease p = 0.09 (Table 5).
Table 5 Comparison between the two groups regarding the number of grafts
| Number of grafts | AF | Chi-Square | ||||||
|---|---|---|---|---|---|---|---|---|
| Group I (POAF) | Group II (NSR) | Total | ||||||
| n | % | n | % | n | % | X2 | p-value | |
| One | 1 | 4.55 | 12 | 15.38 | 13 | 13.00 | 1.867 | 0.393 |
| Two | 12 | 54.55 | 40 | 51.28 | 52 | 52.00 | ||
| Three | 9 | 40.91 | 26 | 33.33 | 35 | 35.00 | ||
| Total | 22 | 100.00 | 78 | 100.00 | 100 | 100.00 | ||
Echocardiographic parameters
The mean left ventricular end-diastolic volume (LVEDV) in Group 1 was 80.7 ± 15.2 ml, while the mean left ventricular end-systolic volume (LVESV) was 35.8 ± 16.8 ml. The mean left ventricular ejection fraction LVEF was 58.9 ± 7% with no significant difference than Group 2 (Table 6).
Table 6 Comparison between conventional echocardiography regarding the two groups
| Conventional echocardiographic study | Group 1 (POAF) | Group 2 (NSR) | t-test | ||
|---|---|---|---|---|---|
| t | p-value | ||||
| LVEF | Range | 45 - 72 | 40 - 80 | −0.378 | 0.706 |
| Mean ± SD | 58.909 ± 7.224 | 59.679 ± 8.742 | |||
| EDV | Range | 60 - 110 | 60 - 130 | 0.320 | 0.750 |
| Mean ± SD | 80.727 ± 15.251 | 79.333 ± 18.735 | |||
| ESV | Range | 21 - 80 | 21 - 92 | −0.061 | 0.951 |
| Mean ± SD | 35.864 ± 16.802 | 36.115 ± 17.005 | |||
| Max LA Vol. | Range | 75 - 110 | 63 - 110 | −0.888 | 0.377 |
| Mean ± SD | 94.909 ± 7.628 | 96.744 ± 8.796 | |||
The mean LA maximal volume in Group 1 (POAF) was 94.909 ± 7.628, while the mean LA volume at Group 2 (NSR) was 96.74.
Discussion
Atrial fibrillation found to be the most common serious arrhythmia and is responsible for substantial morbidity and mortality in the general population9.
The purpose of the study was to detect the angiographic parameters for the prediction of post-operative atrial fibrillation in patients with ischemic heart disease undergoing coronary artery bypass graft.
POAF detected most often between the 2nd and 4th post-operative day, with peak incidence was found in post-operative day two10.
In the present study, of the study population, 22 patients developed AF (22%) versus 78 patients remained in sinus rhythm (78%). This result is in agreement with Burrage et al.11 who reported that atrial fibrillation after cardiac surgery (AFACS) is the most common post-operative complication following cardiac surgical procedures and occurs in 25% after isolated coronary artery bypass grafting (CABG), at the (New-Onset Atrial Fibrillation in Adult Patients After Cardiac Surgery) study11.
Atrial fibrillation (AF) prevalence increases with age, making it the most common arrhythmia in patients older than 65 years. For patients older than 80 years, the corresponding rate is approximately 10%. Furthermore, 70% of individuals with AF are between the age of 65 and 85 years12.
However, this is in contrast to results reported by Kazemi et al.13, who studied the right atrial dyssynchrony and atrial fibrillation after coronary artery bypass grafting surgery13. They reported that there was no statistical difference regarding age (p = 0.145); the difference may be due to they exclude the patients with recent infarction (< 1 month).
In the current study, there was no significant statistical difference between the two groups as regards the risk factors (DM, HTN, smoking, and dyslipidemia), p > 0.05.
These results are in agreement with Açıl et al.14, who assessed the value of pre-operative echocardiography in the prediction of post-operative atrial fibrillation following isolated coronary artery bypass grafting. They reported that there were no significant differences between patients with and without POAF as regards the presence of DM (p = 0.973), HTN (p = 0.437), and dyslipidemia (p = 0.689)14.
In the current study, there is no significant difference between the two groups regarding LVEF; this is in agreement with Rasmussen et al.15, who concluded that no conventional measure including LAV differed between the two groups15.
In the current study, there was no significant statistical difference between the two groups regarding left ventricular end-diastolic volume (LVEDV) (80 ± 15.25 vs. 79.3 ± 18.7 ml; p = 0.750) and left ventricular end-systolic volume (LVESV) (35.86 ± 16.8 vs. 36.11 ± 17 ml; p = 0.95). This is in agreement with Açıl et al.14, who reported that there is no statistical difference between the two groups regarding left ventricular end-diastolic volume (LVEDV) (p = 0.874) and left ventricular end-systolic volume (LVESV) (p = 0.907)14.
Our study shows no significant difference between the two groups regarding max LA volume, which agrees with Burrage et al.11. The later studied new-onset atrial fibrillation in adult patients after cardiac surgery that there is no significant difference in that study as regards atrial fibrillation postoperatively11.
This is in contrast with Nardi et al.16 when they study the relationship between POAF and LA volume following CABG, POAF was observed in 61 patients (27.7%). POAF patients showed increased left atrial volume (59.0 ± 18.3 ml vs. 70.6 ± 28.1 ml; p = 0.0004). Left atrial volume was an independent risk factor for POAF in that study. This study was highly selected. Inherent selection bias could limit the generalization of the results to all patients undergoing CABG. Cardiac surgeons and practitioners in the ICU were not blinded to echocardiography results. Thus bias could arise from potential differences in drug treatment according to the presence or absence of echocardiographic evidence of LA enlargement16.
In our study, there is a significant difference (0.014) between the two groups as regards the left main (LM) disease, patients with the left main disease have a high probability of having POAF after CABG than other diseases.
Our result goes with Petre et al.17, which concluded that New-Onset Atrial Fibrillation (NOAF) was common after CABG but extremely rare after PCI in patients with LMCAD undergoing revascularization in (the EXCEL trial). Among 1812 patients without atrial fibrillation on presentation, NOAF developed at a mean of 2.7 ± 2.5 days after revascularization in 162 patients (8.9%), including 161 of 893 (18.0%) of CABG-treated patients and 1 of 919 (0.1%) of PCI-treated patients (p < 0.0001)17.











 nueva página del texto (beta)
nueva página del texto (beta)