Introduction
The anoles (Iguanidae, Dactyloinae) are a group of lizards with nearly 400 species naturally distributed from the United States of America through Central America and the Caribbean to Bolivia, with naturalized populations in Asia (Nicholson et al., 2012; Poe et al., 2017; de Queiroz et al., 2020). Forty-four anole species occur in Ecuador and to date there are few records of parasites (Torres-Carvajal et al., 2022). The knowledge of parasitic fauna of more anole species may provide important information to better understand their diet, habitat use and biological interactions with other organisms (Marcogliese, 2004; Pereira et al., 2013). Parasites can influence competitive and predatory interactions between species by reducing the competitive strength of infected hosts against a non-host species (Hatcher et al., 2006). Helminths such as nematodes can cause low food intake, reduce growth, and increase mortality of the hosts (Goater & Ward, 1992). In addition, helminth dominance patterns and anoles species composition show that habitat differences are determining factors for the infection of these parasites (Bundy et al., 1987). Arthropods can affect the behavior and the energy metabolism of lizards (Schall & Sarni, 1987), the outcome of interspecific competition among anoles (Schall, 1992) and decrease the survival of parasitized individuals (Irschick et al., 2006).
The present checklist summarizes the diversity of parasites in the anoles of Ecuador found in literature and in six species of anoles reviewed in the laboratory as a basis for providing a host-parasites list needed for future work and to understand their ecology.
Materials and methods
This checklist was prepared based on records of mites, dipterans and helminths from literature and was organized based on the taxonomic categories and the name of the authority who described each taxon and year, as well as new material examined in Ecuador. The taxonomy of the parasites follows the references in the literature, as well as nematodes (Ávila & Silva, 2010; Boada, 2015; Morand et al., 2015), platyhelminthes (Ávila & Silva, 2010), mites (Walter & Proctor, 2013); and dipterans (Florez & Wolff, 2009; Hall, 2013). Taxonomy of hosts is reviewed based on recent publications (de Queiroz et al., 2020; Torres-Carvajal et al., 2022; Uetz et al., 2022). Each record contains the phylum, class, order, family and species of the parasites. In addition to its host(s), site of infection and geographical record (state/province when available) are provided. Comments include specimens of literature reviewed and changes in the host taxonomy.
We examined six species of AnolisDaudin, 1802 from Ecuador deposited in the herpetology collection of the Zoology Museum of the Pontificia Universidad Católica del Ecuador (QCAZR): Anolis draculaYánez-Muñoz et al., 2018 (QCAZR 14888) from Goaltal, Carchi Province (0.82111° N, 78.13900° W, 1,542 m a.s.l.); A. nemonteaeAyala-Varela et al., 2021 (QCAZR 14597) from Buenaventura Reserve, El Oro Province (3.65061º S, 79.78050º W, 372 m a.s.l.); A. gracilipesBoulenger, 1898 (QCAZR 10693) from Centro de Interpretación Ambiental Otongachi, Unión del Toachi, Pichincha Province (0.321389º S, 78.95150º W, 836 m a.s.l.); and A. parvauritusWilliams, 1966 (QCAZR 12191) from Canandé Reserve, Esmeraldas Province (0.52069º N, 79.21438º W, 401 m. a. s. l. ); A. trachydermaCope, 1875 (QCAZR 11745) from Lorocachi, Pastaza Province (1.656694º S, 75.96980º W, 185 m a.s.l.); and A. scypheus Cope, 1864 (QCAZR 14789) from Saladero to 1.8 km N Río Yasuní, Yasuní National Park, Orellana Province (0.92100º S, 75.96358° W, 229 m a.s.l.).
The ectoparasites obtained from the lizards were extracted with forceps and the endoparasites were separated by dissection of the specimens. The parasites were deposited in the invertebrate collection (QCAZI) of the same institution. Taxonomic keys were used to identify the parasites (FAO, 1990; Hall, 2013; Florez & Wolff, 2009; Guimarães & Papavero, 1999; Serrano, 2010; Walter & Proctor, 2013; Morand et al., 2015). The parasites were identified to family and some to genus level because the larval stages have not yet matured their important characters to distinguish species.
Specimens were photographed with the Steresocope ZEISS Stemi SV6 and inverted ZEISS TELAVAL 31 microscope. Measurements and digital analysis were performed with a Lumenera microscopy camera (model Infinity 1-M1, Canada) and Lumenera’s Infinity Analyze and Capture software® (Lumenera Corporation, 2016). Reference specimens from the QCAZ Invertebrate Museum were also used to corroborate the information obtained from the identification of nematodes.
Results
We compiled a list of 11 taxa of parasites and 9 species of hostanoles (Table 1) obtained from the literature and this study. In this study, we found three invertebrate families (Ascarididae, Trombiculidae and Calliphoridae) and 25 specimens of parasites (Table 2). The endoparasites recorded are nematodes of the family Ascarididae in anoles as Anolis dracula, A. trachyderma and A. scypheus. Among the recorded ectoparasites are larvae of the genus CochliomyiaTownsend, 1915 of the family Calliphoridae in Anolis parvauritus; and mites of the family Trombiculidae in Anolis nemonteae and A. gracilipes.
Tabla 1 Lista de hospederos-parásitos de los anolis del Ecuador.
| Host | Parasite |
|---|---|
| Anolis dracula | Ascarididae gen. sp. |
| Anolis fuscoauratus | Cosmocerca vrcibradici |
| Anolis fuscoauratus | Strongyluris oscari |
| Anolis fuscoauratus | Rhabdias sp. |
| Anolis fuscoauratus | Oswaldocruzia bainae |
| Anolis fuscoauratus | Oswaldocruzia vitti |
| Anolis gracilipes | Trombiculidae gen. sp. |
| Anolis nemonteae | Trombiculidae gen. sp. |
| Anolis parvauritus | Cochliomyia sp. |
| Anolis punctatus | Strongyluris oscari |
| Anolis punctatus | Rhabdias elegans |
| Anolis punctatus | Rhabdias sp. |
| Anolis punctatus | Oswaldocruzia vitti |
| Anolis purpurescens | Nematoda |
| Anolis scypheus | Ascarididae gen. sp. |
| Anolis trachyderma | Ascarididae gen. sp. |
| Anolis trachyderma | Cairaella henrii |
Tabla 2 Parásitos de Anolis examinados en laboratorio, número de individuos, estadio y hábitat.
| Host | Parasites | No. Individuals | Habitat | Stage |
|---|---|---|---|---|
| Anolis dracula | Ascarididae | 6 | Thoracic cavity | Adult |
| Anolis gracilipes | Trombiculidae | 3 | Dewlap | Larvae |
| Anolis nemonteae | Trombiculidae | 1 | Dewlap | Larvae |
| Anolis parvauritus | Cochliomyia sp. | 8 | Thoracic cavity, stomach, liver, pelvic cavity | Larvae second instar |
| Anolis trachyderma | Ascarididae | 6 | Thoracic cavity | Adult |
| Anolis scypheus | Ascarididae | 1 | Thoracic cavity | Adult |
The specimens of the family Ascarididae (nematodes) are characterized by having a vermiform, cylindrical, nonsegmented body, a slightly curved tail and three cephalic lips around the mouth, the average length of the individuals analyzed is 10 mm (10,000 µm).
The specimens of the family Trombiculidae (mites) are characterized by having 6-legged, round-shaped larvae of small size 0.1 mm (100 µm) not yet pigmented, poorly sclerotized with no external genitalia. The gnatosome is located in the middle of the first pair of legs, coxas I and II united; ventrally the idiosome shows very small setae while the legs, including the tarsi (final segments) show more defined setae.
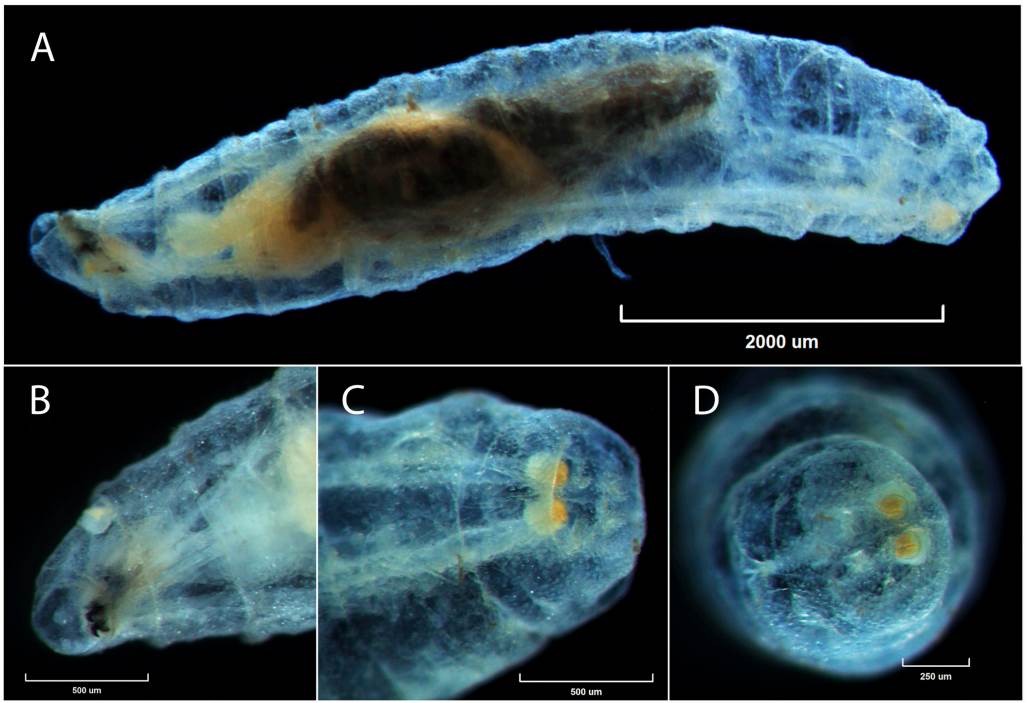
The specimens of Cochliomyia, Calliphoridae (Diptera) belong to second-instar. On average they measured 6 mm in length (6,000 µm ± SD), presented a fusiform body (Fig. 1A), posterior margin of segment 11 without spines and not well developed and sclerotized cephalopharyngeal skeleton (Fig. 1B), band of body spines with 2 tips, anterior spiracle from 9 to 11 gills, tracheal trunks with very light pigmentation in the 12th segment (Fig. 1C), posterior spiracle with incomplete peritrema lightly pigmented and imperceptible button (Fig. 1D).
List of parasites
PHYLUM NEMATODA
Host: Anolis purpurescens Cope, 1899 Site of infection: Intestine.
Site of infection: Intestine.
Distribution: Pichincha.
Comments: Reported in Boada (2015).
CLASS CHROMADOREA
ORDER ASCARIDIDA
FAMILY ASCARIDIDAE gen. sp.
Host: Anolis dracula Yánez-Muñoz et al., 2018 (QCAZR14888)
Site of infection: thoracic cavity.
Distribution: Carchi, Goaltal.
Comments: New material examined QCAZI261046.
Host: Anolis trachyderma Cope, 1875 (QCAZR11745)
Site of infection: cloaca.
Distribution: Pastaza, Lorocachi.
Specimens deposited: QCAZI261050
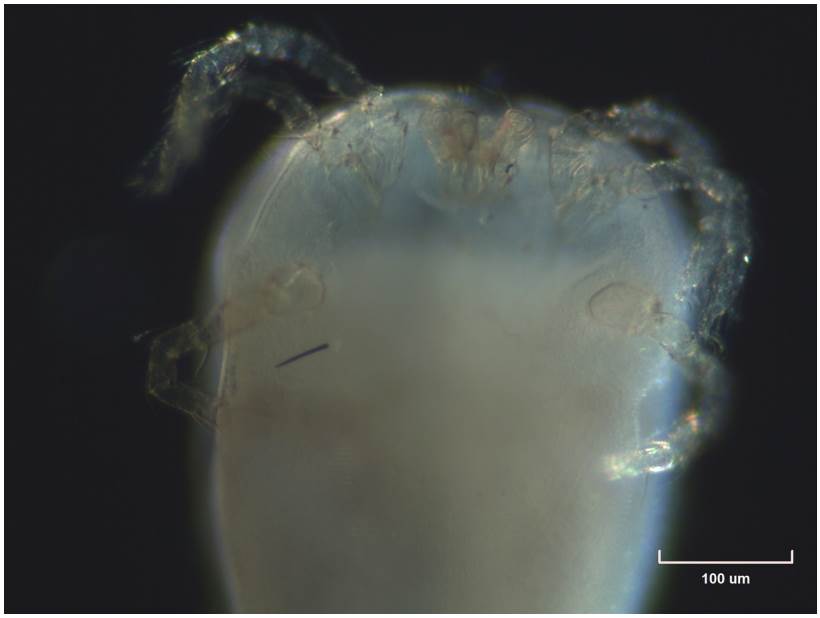
Host: Anolis scypheus Cope, 1864 (QCAZR14789)
Site of infection: thoracic cavity.
Distribution: Orellana, Parque Nacional Yasuní, Río Yasuní.
Commenst: New material examined QCAZI261047 (Fig. 2).

FAMILY COSMOCERCIDAE
GENUS CosmocercaDIESING, 1861
Cosmocerca vrcibradici BURSEY AND GOLDBERG, 2004
Host: Anolis fuscoauratus D’Orbigny, 1837
Site of infection: Intestine.
Distribution: Sucumbíos.
Comments: Reported in Ávila & Silva (2010).
CLASS SECERNENTEA
ORDER ASCARIDIDA
FAMILY HETERAKIDAE
GENUS Strongyluris MUELLER, 1894
Strongyluris oscari TRAVASSOS, 1923
Host: Anolis fuscoauratus, A. punctatus Daudin, 1802, A. transversalisDuméril & Duméril, 1851.
Site of infection: Stomach, intestine.
Distribution: Sucumbíos and Pastaza.
Comments: Reported in Goldberg et al. (2006), McAllister et al. (2010), and Ávila & Silva (2010).
ORDER RHABDITIDA
FAMILY RHABDIASIDAE
GENUS Rhabdias STILES AND HASSALL, 1905
Rhabdias elegans GUTIERREZ, 1945
Host: Anolis punctatus
Site of infection: Lungs.
Distribution: Pastaza.
Comments: Reported in McAllister et al. (2010).
Rhabdias sp.
Host: Anolis fuscoauratus, A. punctatus.
Site of infection: Lungs, stomach.
Distribution: Sucumbíos.
Comments: Reported in Goldberg et al. (2006) and Ávila & Silva (2010).
ORDER STRONGYLIDA
FAMILY MOLINEIDAE
GENUS Oswaldocruzia TRAVASSOS, 1917
Oswaldocruzia bainae BEN-SLIMANE AND DURETTEDESSET, 1996
Host: Anolis fuscoauratus, A. scypheus.
Site of infection: Intestine.
Distribution: Sucumbíos.
Comments: Reported in Ávila & Silva (2010). Oswaldocruzia vittiBURSEY AND GOLDBERG, 2004
Host: Anolis fuscoauratus, A. punctatus.
Site of infection: Intestine.
Distribution: Sucumbíos.
Comments: Reported in Goldberg et al. (2006) and Ávila & Silva (2010).
PHYLUM PLATYHELMINTHES
CLASS CESTODA
ORDER ONCHOPROTEOCEPHALIDEA
FAMILY PROTEOCEPHALIDAE
GENUS Cairaella COQUILLE AND DE CHAMBRIER, 2008
Cairaella henrii COQUILLE AND DE CHAMBRIER, 2008
Host: Anolis trachyderma Cope, 1876
Site of infection: Intestine.
Distribution: Sucumbíos.
Comments: Reported in Avila & Silva (2010).
PHYLUM ARTHROPODA
CLASS EUCHELICERATA
ORDER TROMBIDIFORMES
FAMILY TROMBICULIDAE gen. sp.
Host:Anolis nemonteae (QCAZR14597)
Site of infection: dewlap.
Distribution: El Oro, Reserva Buenaventura.
Comments: New material examined QCAZI261048.
Host: Anolis gracilipes Boulenger, 1898 (QCAZR10693):
Site of infection: dewlap.
Distribution: Pichincha, Centro de Interpretación Ambiental Otongachi.
Comments: New material examined QCAZI261051 (Fig. 3).
CLASS INSECTA
ORDER DIPTERA
FAMILY CALLIPHORIDAE
GENUS Cochliomyia sp.
Host: Anolis parvauritus Williams, 1966 (QCAZR12191)
Site of infection: internal damage in abdominal and pelvic region.
Distribution: Esmeraldas, Reserva Canandé.
Comments: Larvae report in Narváez et al. (2019) like Chrysomya sp. Robineau-Desvoidy, 1830 (Calliphoridae), QCAZI261049 (Fig. 1).
Discussion
We report for the first time, mites and nematodes found in three species of Anolis of the western slopes of Ecuador (A. dracula, A. gracilipes and A. nemonteae). Most reports of parasites in Ecuador are for Amazonian host species (A. fuscoauratus, A. punctatus, A. scypheus, and A. trachyderma). We also report a new family of Nematoda for A. trachyderma. which indicates the importance of characterizing taxonomically the parasites of all species of anoles.
Reptiles are hosts to a wide variety of nematodes, many of which inhabit the digestive tract or lungs (Jacobson, 2007). Nematodes of the family Ascarididae have high prevalence rates in lizards of the genus Anolis and exhibit the greatest diversity in relation to the specificity with these lizards. Therefore, there is a great opportunity to test hypotheses about the diversification of parasites in these lizards (Morand et al., 2015).
Mites of the family Trombiculidae were not identified to genus or species because they lack further development of their characters. These mites are the only ones parasitizing vertebrate hosts during their larval instar (Walter & Proctor, 2013).
Larvae of the family Calliphoridae cause cutaneous myiasis (FAO, 1990). We reviewed the larvae reported by Narvaez et al. (2019) that were identified as belonging to the genus Crysomya. However, taxonomic re-identification showed that they belonged to the genus Cochliomyia as Carrillo (2015) points out that the presence of bands of small spines with 1 to 2 tips in the body of members of Cochliomyia is a diagnostic trait of the genus, while Florez & Wolff (2009) mention the presence of up to 3 tips in the body spines of Chrysomya.
The peritreme is slightly pigmented in Cochliomyia while Chrysomya shows a marked pigmentation. In addition, Carrillo (2015) states that in the late larval stages of Cochliomyia presents two tracheal trunks that can be pigmented from the 9th to 12th segment, even if pigmentation can be found only up to 1/3 of the 12th segment.
On the other hand, in early larval stages this pigmentation is very light. In addition, Guimarães & Papavero (1999) indicate that the genus Cochliomyia does not have spines on the posterior margin of segment 11 while the genus Chrysomya does have spines on this body segment.
The larvae of the family Calliphoridae were not found in an external wound in the lizards of the genus Anolis, because wounds are scarce in natural conditions, the female Cochliomyia must lay her eggs at the edge of the natural orifices of favorably exposed vertebrates (Forero et al., 2008).
Because knowledge of the immature stages of Calliphoridae species in South America is poor, it is important to rear the larvae to adults for effective species identification following the FAO (1990) manual.
Parasites compete with their hosts for resources, causing damage in many ways (Price, 1980). However, parasitism has an important role in ecosystems, regulating the abundance or density of host populations, stabilizing food chains and structuring animal communities (Zaman et al., 2014).
Zaman et al. (2014) considers that host parasite interaction causes a co-evolution where parasites force their hosts to become more complex to avoid extinction. Therefore, parasites are important promoters and protectors of biological diversity because antagonistic interactions and natural selection together increased complexity and evolvability.
We think that comparative studies among different anole populations are necessary to determine the most important factors that establish the composition and structural distribution of the parasites in these lizards, and to understand the infection levels and the effects of the parasites on their ecology and survival.
The present work is an important contribution to the knowledge of parasites in Anolis. It is necessary to study the parasitology associated with Anolis, since the current situation demands not only the knowledge of its taxonomy but also monitoring studies that allow understanding the ecological impacts on the populations of this host genus.











 nueva página del texto (beta)
nueva página del texto (beta)