Introduction
Livestock farming is one of Mexico’s most important economic activities, and the country is ranked seventh in the world as a powerhouse in the generation of livestock products (FAO 2020). Livestock is a cost-effective sector that guarantees the production and supply of accessible, safe and quality food (Figueroa-Antonio et al. 2018). The livestock sector is affected by different diseases caused by parasites, such as gastrointestinal nematodes (GIN), which represent one of the most important health problems in livestock worldwide (Pinilla et al. 2018). Sub-clinical infections and the presence of GIN are determined by biotic and abiotic factors, such as climate, animal management and the age of hosts exposed to contaminated pastures (Byron et al. 2018). Parasitism caused by GIN causes economic losses in Mexico estimated at 35 billion pesos per year (FAO 2020). The economic losses are mainly caused by a decrease in milk and meat production and an increase in the costs associated with treatment and control of GIN (Pinilla et al. 2018).
Given this problem, it is essential to carry out studies to identify sustainable alternatives to control highly prevalent gastrointestinal parasites, as well as to understand their epidemiology and susceptibility to anthelmintic drugs (Byron et al. 2018).
Chemical products (e.g., macrocyclic lactones) used in an inadequate and constant manner can cause anthelmintic resistance (Lumaret et al. 2012, Márquez-Lara 2013). Additionally, the use of these chemical products threatens the environment (Martínez and Cruz 2009). Medicines, such as fenbendazole and oxfendazole, are excreted by ruminants in large quantities and put beneficial organisms, such as dung beetles (Onthophagus landolti and Aphodius rufipes), earthworms and nematophagous mites (Caloglyphus mycophagus), at risk (Aguilar-Marcelino et al. 2016, Quintero 2018).
Biocontrol can serve as an alternative to chemical products (Al-ani et al. 2020). Fungi are antagonists of parasitic nematodes of vertebrate animals, plants, insects and other pathogenic fungi (Fernández-Jiménez et al. 2019). They are excellent biocontrol agents due to their high reproductive capacity, specificity, production of resistant spores or development of saprophytic phases in the absence of their hosts, and antagonistic activities (Fernández-Jiménez et al. 2019). Barron and Thorn (1987) reported the mechanism of attack of P. ostreatus against the free-living nematode Rhabditis spp. Initially, the mycelium of the fungus produces toxocysts, which are mainly composed of a nematotoxin called ostreanin, to attract the nematodes. Once the nematodes have made contact with the ostreanin, they become immobilized and the mycelium quickly penetrates their body through the oral cavity, anus and intercuticular spaces, allowing the fungus to subsequently feed on the nematode. The species of Pleurotus that showed this nematicidal activity are P. strigosus, P. subareolatus and P. cornucopiae. In addition, Li and Zhang (2014) reported 23 species of the genus Pleurotus with nematicidal activity. Based on the information that has been described to date, the objective of the present review was to analyze the characteristics of the genus Pleurotus as a biocontrol agent of parasites of importance for livestock.
Parasites Of Importance To Livestock
A parasite is an organism that lives in another organism from which it feeds (SENASA 2017, Gutiérrez et al. 2020). Animals can become infected with parasites and can spread the infection. The animal on which the parasite feeds is called the host. The main helminths include the hematophagous nematode (Haemonchus contortus), cestodes (Taenia saginata) and trematodes (Fasciola hepatica) (SENASA 2017, Gutiérrez et al. 2020).
The GIN are found within the organs of the digestive tract, where copulation takes place between males and females, which produces eggs that are disposed of into the environment along with the animal’s feces (Sutherland and Scott 2010). There are also other parasites that cause intestinal trichostrongylosis. The most important genera from a veterinary point of view are Trichostrongylus, Cooperia and Nematodirus (Von son-De-Fernex et al. 2016, Pinilla et al. 2018).
Gastroenteric parasitosis in ruminants mainly bovines and sheep are very important due to their cosmopolitan nature, causing great economic losses that can be direct such as the death of young animals or indirect that are clinically manifested by persistent diarrhea, anemia and malnutrition, resulting in stunted growth in young animals, as well as low production of meat and milk in adult animals (Valcárcel-Sancho et al. 2009, Liébano et al. 2011). In Table 1, the most important gastrointestinal parasites (nematodes) of ruminants are presented.
Table 1 Gastrointestinal parasites (nematodes) of ruminants
| Species | Organ |
| Haemonchus contortus | Abomasum |
| H. placei | Abomasum |
| Mecistocirrus digitatus | Abomasum |
| Trichostrongylus axei | Abomasum |
| T. colubriformis | Abomasum |
| T. longispicularis | Abomasum |
| Teladorsagia/Ostertagia circumcincta | Abomasum |
| O. trifurcata | Abomasum |
| O. lyrata | Abomasum |
| Nematodirus spathiger | Small intestine |
| N. fillicolis | Small intestine |
| N. helvetianus | Small intestine |
| N. battus | Small intestine |
| Strongyloides papillosus | Small intestine |
| Bunostomum trigonocephalum | Small intestine |
| B. phlebotomum | Small intestine |
| Cooperia oncophora | Small intestine |
| C. pectinata | Small intestine |
| Trichostrongylus spp. | Small intestine |
| Toxocara vitulorum | Small intestine |
In rural areas of the country, animal husbandry is the main activity that supports the economy of producers and their families (López-González et al. 2012). However, this activity is carried out under precarious conditions that make the animals vulnerable to endemic diseases such as parasites; this has caused economic losses estimated at 35 billion pesos due to reductions in animal performance, deterioration of product quality and increased costs of prevention, control, treatment and animal death (FAO 2020). Parasitic diseases affect livestock production, as well as public health, and their risks are associated with production systems (SENASA 2017).
Parasites are very diverse and may vary from one region to another; however, the genera commonly found in Mexico are Haemonchus, Trichostrongylus, Cooperia, Strongyloides, Nematodirus, and Oesophagostomum (Rodríguez-Vivas et al. 2017).
Helminths are found in different climates thanks to their high reproductive capacity and various adaptation mechanisms in response to adverse environmental conditions. Parasite species in cattle prefer certain organs (Paredez-Martinez 2014). These parasites have a direct life cycle, with a free-living phase in the pasture and a parasitic phase in the animal. Most of these parasites infect animals via the oral route following ingestion of infectious larvae (L3) present in water and food. Some factors favor the development of the parasites in the free-living phase, including humidity above 80 % and temperatures between 25-27 ºC, which facilitate the evolution of the larvae over 7-10 days. Eggs are excreted with sheep feces into the grass, there they hatch into the larval stage and some migrate to pastures in water films, while others remain on the damp surface of the ground and manage to survive for months. In the dry season, larvae prefer wet sites because drying is detrimental to their survival (Paredez-Martinez 2014).
Haemonchus contortus
Haemonchus contortus is a nematode with blood-sucking habits, and it has 16 intestinal cells and a protective sheath in its infective stage (L3) (Zajac and Garza 2020). While H. contortus is more prevalent in tropical climates, it is also present in various parts of the world and has a negative impact on the livestock sector (Widiarso et al. 2018).
Life cycle of Haemonchus contortus
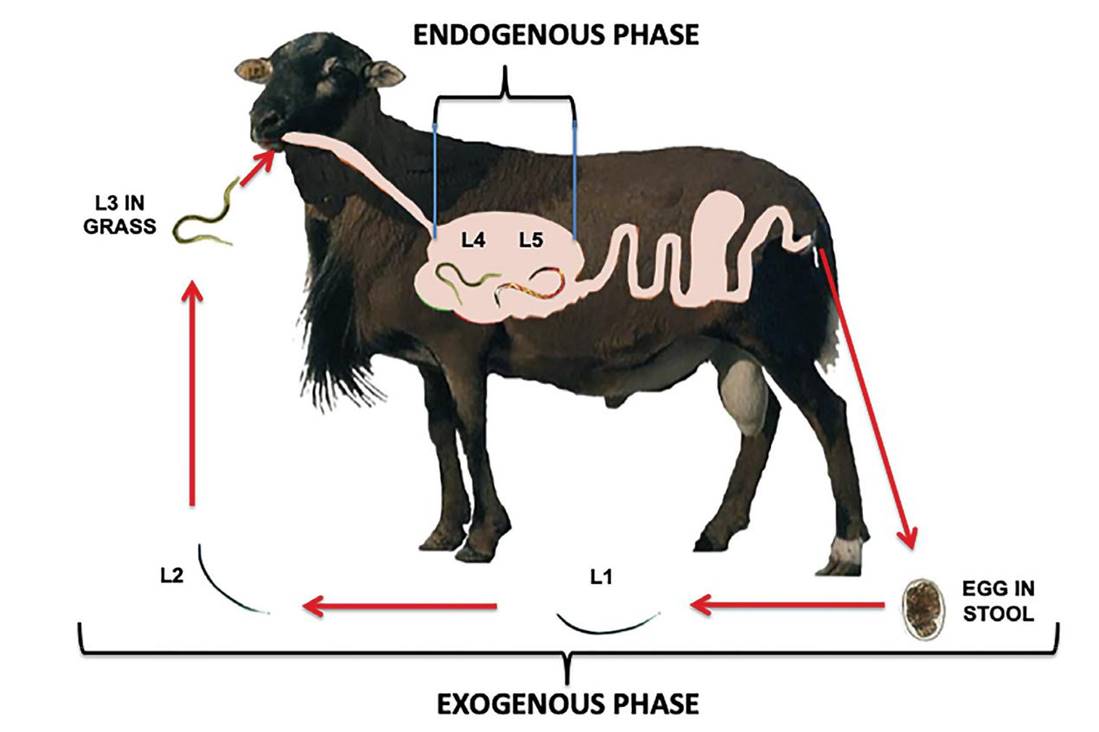
The life cycle of the sheep parasitic nematode H. contortus is divided into two phases: 1) exogenous and 2) endogenous. The exogenous phase begins when the sheep expel the eggs into the pasture in their feces; under optimal conditions, the L1 larvae develop. This occurs in 1-2 days, after which the eggs hatch and the free-living larvae (L2) emerge. After seven days, they reach their infective stage, known as L3. These larvae migrate to the upper parts of the pastures to be ingested by the animals along with the grass (Zajac and Garza 2020). The endogenous phase starts when the infectious larvae (L3) have been ingested by the sheep, at which time it enters the digestive tract and develops into L4 and later, into L5. In this last stage the nematode is considered an adult (Sutherland and Scott 2010). It should be mentioned that stages L4 and L5 feed on blood and tissue, and this activity irritates the mucosa causing inflammation. In addition, as the nematodes extract considerable amounts of blood, anemia develops, which is manifested as paleness of the conjunctiva and gums. At this stage, the animals become indifferent and suffer from low food conversion, their hair becomes brittle and dull, and the animals lose their appetite, rendering them unable to regain their normal weight, sometimes resulting in death (Gasque 2008) (Figure 1).
Pest control in the livestock arena
These parasites have traditionally been treated with regular administration of chemical drugs (anthelmintics) as a simple and “effective” way to alleviate parasitic diseases. However, many animals have high parasite loads and clinical features of parasitosis (Lobayan et al. 2017), as the parasites have developed resistance to the anthelmintic products administered (González-Garduño et al. 2003, Torres-Acosta et al. 2003, Lanusse et al. 2018).
Anthelmintic products are currently the main method used to control ruminant nematodes worldwide. They have different mechanisms of action, and avermectins, benzimidazoles and nicotinic agonists are the most common anthelmintics used in ruminants (Márquez-Lara 2013).
Ivermectins are one of the anthelmintics available and they are frequently used by producers. However, they have negative effects on the environment, especially on microfauna such as beneficial arthropod populations associated with feces (they mainly affect their larval forms) (Márquez-Lara 2013).
Anthelmintic resistance can be either intrinsic or acquired; in the former, a parasite is naturally resistant to an anthelmintic due to the absence of receptors or the inability of the product to enter the site of action, as occurs in the resistance of trematodes and cestodes to macrocyclic lactones. Acquired resistance occurs in parasites that were originally susceptible to the drug, but after genetic modifications of the parasite that become hereditary, they are no longer susceptible (Márquez-Lara 2013, Olazarán-Jenkins et al. 2019).
Sustainable alternatives: edible mushrooms
An alternative method for parasite control in the livestock arena is edible mushrooms. Ascomycete, basidiomycete and zygomycete fungi have developed various strategies to attack nematodes, which are the most abundant organisms in the soil. They have developed these mechanisms to obtain the nitrogen requirements (van den Hoogen et al. 2019). In fact, Hyde et al. (2014) was the first to report a fungus with the capacity to trap nematodes corresponding to the species Palaeoanellus dimorphus, which lived 100 million years ago and are probably extinct today. Currently, fungi with nematicidal activity are distributed throughout the world. According to Li and Zhang (2014), there are 280 species belonging to 150 genera between the ascomycetes and basidiomycetes that have nematicidal capacity.
Barron and Dierkesy (1977) established that some species of Hohenbuehelia, and its anamorph Nematoctonus, had anthelmintic capacity. Furthermore, Barron and Thorn (1987) reported that the genus Pleurotus was more versatile than Hohenbuehelia because in addition to nematodes, other organisms could also serve as nutrients.
On the other hand, there are also nematodes that parasitize edible mushrooms and cause serious problems in their development and productivity. The most documented case is Agaricus bisporus, although the genus Pleurotus is not exempt from their attack (Belletini et al. 2018, Singh and Sharma 2016). Finally, there is also the case of beneficial nematodes for edible mushrooms that, being antagonists of their pests, are useful in biological control strategies (Choo et al. 2001, Rinker et al. 1995).
Classification of nematophagous fungi
Fungi are organisms that possess the ability to attack, kill and digest the body of nematodes (Rodríguez-Martínez et al. 2018). It has been observed that the mycelium of edible fungi can infect nematode eggs (Nordbring-Hertz et al. 2006). In addition to their nematicidal ability, these fungi can also live saprophytically on dead organic matter, and they can attack other fungi (mycoparasites) and colonize plant roots as endophytes (Nordbring-Hertz et al. 2006). These fungi described below (Hyde et al. 2014), are divided into four groups depending on their mechanism of action. The nutrients obtained by the fungi from the nematodes are used to form new fungal structures.
1) Trap forming fungi
These fungi form various types of trapping organs in their hyphae. There are two different mechanisms that function in the traps: adhesive and mechanical. Whatever the mechanism, the fungus can penetrate the cuticle of the nematode through the trap, forming a bulb of infection inside the nematode, from which the trophic hyphae grow inside the body and digest its contents (Nordbring-Hertz et al. 2006, Saumell et al. 2008, Mendoza-de-Gives and Valero-Cross 2009, Castañeda-Ramírez et al. 2016, Aguilar-Marcelino et al. 2017a, Mendoza-de-Gives 2020).
2) Endoparasitic fungi
Endoparasites use their spores to infect nematodes. These fungi are often forced parasites of nematodes, and they appear only as structures of dissemination outside the infected body of the nematode. The spores of these fungi adhere to and penetrate the cuticle of the nematode, thereby becoming embedded in it (Nordbring-Hertz et al. 2006, Zhang et al. 2011).
3) Ovicidal fungi
These fungi infect nematode eggs and produce appressoria (infection structures at the ends of the hyphae that attach to the egg cover). The fungi penetrate the egg cover and digest the contents (Nordbring-Hertz et al. 2006).
4) Toxin-producing fungi
The most common fungus in this group is the wood decomposer P. ostreatus, along with other species of the genus Pleurotus. This type of fungus contains a toxin in its hyphae; after contact with the toxin, the nematode is quickly immobilized. The fungal hyphae then grow chemotropically through the mouth of the nematode, digesting it, similar to the nematophagous fungi (Nordbring-Hertz et al. 2006).
The majority of these fungi belong to the basidiomycetes, for example, the genera Pleurotus and Coprinus; however, other fungi within the ascomycetes (Lecanicillium, Paecilomyces, and Pochonia) also produce nematicidal compounds. The fungus Paecilomyces lilacinus secretes acetic acid that paralyses young nematodes (Djian et al. 1991), and bioactive compounds have also been isolated from in vitro cultures of Pochonia chlamydosporia. Currently, more than 270 species of fungi have been reported to produce nematicidal compounds (Zhang et al. 2011).
Coprinus comatus has been reported to immobilize the activity of Panagrellus redivivus via two strategies (Luo et al. 2007). The first is through spiny spherical structures (toxocysts) (Armas-Tizapantzi et al. 2019) that attract nematodes; when the toxocysts come into contact with the cuticle, they immobilize the nematode and the fungal mycelia invades the body and degrades it (Barron and Thorn 1987, Al-ani et al. 2020).
The second strategy involves the production of various products including toxins, such as ostreatin/ trans-2-decanoacid, linoleic acid peroxide and extracellular proteases (serine proteases) (Kwok et al. 1992); proteins (Cuevas-Padilla 2019), such as ostreolysin, amatoxin, coprin, phaloxin and acromelic acid; and secondary metabolites, including cheimonophyllon E and 5-hydroxymethyl-furancarbaldehyde in P. ferulae, trans-2-decenedioic acid from P. ostreatus, and p-anisaldehyde, p-anisyl alcohol, 1-(4-methoxyphenyl)-1,2-propanediol, 2-hydroxy-(4’-methoxy)-propiophenone, S-choriolic acid and linoleic acid from P. pulmonarius (Cuevas-Padilla et al. 2018, Pineda-Alegría et al. 2017, 2020, Castañeda- Ramírez et al. 2020).
Important Aspects Of The Genus Pleurotus
Pleurotus spp
White rot fungi, such as Pleurotus species, are considered primary decomposition agents because they are able to use agricultural waste in its original form (i.e., not previously subjected to any biochemical or microbiological degradation process) (Camacho-Morales et al. 2017). Several species of edible mushrooms are currently cultivated on an industrial scale. This provides an excellent alternative use for these fungi, as they grow abundantly on coffee pulp (Coffea arabica), sugar cane bagasse (Saccharum officinarum) and henequen (Agave fourcroydes), among other diverse lignocellulosic materials (Salmones and Mata 2012, Sánchez and Royse 2017).
Pharmacological studies of the genus Pleurotus have been carried out on different parts of the fungus, such as the fruiting body, also known as basidiocarp, carpophore or sporophore, and the mycelium, as well as from the degraded substrate. Different extracts and metabolites, such as phenolics, polypeptides, terpenes, sterols and polysaccharides, among others, have been obtained from the fungal materials described above (Ribeiro et al. 2006). Among these metabolites, the most studied are the polysaccharides obtained from the fruiting bodies and mycelia of several species, including P. ostreatus, P. sajorcaju and P. citrinopileatus (Gómez-Velázquez 2018).
Species of genus Pleurotus have different medicinal and therapeutic properties including anti-inflammatory (Smiderle et al. 2008, Gómez-Velázquez 2018), antihypertensive, cardiotonic (Gómez-Velázquez 2018), antiviral, antimicrobial, cytotoxic, antitumor, anticarcinogenic and antioxidant activities (Sánchez et al. 2015). Metabolites, such as lovastatin and others of phenolics, flavonoids and tannins, have been identified and isolated from edible wild mushrooms including P. ostreatus and P. djamor. Of the main phenolic compounds found in the genus Pleurotus, those derived from benzoic acid (gallic and syringic acid) and cinnamic acid (ferulic and caffeic acid) stand out (Oropeza 2017).
Based on their immunomodulatory activity, the fungi of the genus Pleurotus have been further studied as a natural source of bioactive compounds capable of complementing or stimulating a desired immune response in the host. These products include substances of high molecular weight, mainly polysaccharides of the type β-(1,3)-(1,6)-D-glucans, proteins, proteoglycans and polysaccharide-protein complexes, as well as different secondary metabolites of low molecular weight (Quevedo et al. 2018).
Insecticide activity has also been reported. Extracts from the species P. ostreatus have insecticidal properties against Tribolium castaneum and Macrosiphum rosae, with higher insect toxicity observed as the time of extract exposure increases (Pérez-Moreno et al. 2010). Another property of P. ostreatus is its ability to biodegrade organophosphate insecticides in the soil. This is because it secretes several extracellular enzymes that degrade a great variety of natural and anthropogenic compounds, which have chemical structures similar to lignin (Pino et al. 2019). Pleurotus ostreatus also has antiparasitic and anthelmintic (nematicide and cestocides) properties (Pérez-Moreno et al. 2010, Salmones and Mata 2012, Aguilar-Marcelino et al. 2017 b).
Studies carried out by Thorn and Barron (1984) mention five species of Pleurotus, Pleurotus strigosus, P. subareolatus, P. cornucopiae, P. cystidiosus and P. ostreatus, which each present a different capacity to attack and consume nematodes, suggesting that these fungi use the nutrients of their prey (nematodes) to complement the low levels of nitrogen available in wood. This mode of obtaining nutrition is similar in principle to that of the superior “carnivorous” plants. Hibbet and Thorn (1994) also indicated that the genus Pleurotus, sensu Singer, displayed a typical form of nematode capture that seemed characteristic of the genus; they suggested that this could be adequate for identifying members of the genus. They mention the positive activity of the species P. cornucopiae, P. cystidiosus, P. levis, P. ostreatus, P. populinus, P. subareolatus, P. pulmonarius, P. dryinus, P. euosmus, and P. eryngii. Additionally, Sharma (1994) reported that the breeding ground of Pleurotus sajorcaju can immobilize the nematodes of the mushroom Agaricus bisporus and more recently, the species P. djamor has also been reported to have anthelmintic activity (Pineda-Alegría et al. 2017).
The fungus P. tuberregium is a common basidiomycete found in tropical climates in Africa and Australia. Studies show that the sclerotia of this fungus are formed under the soil because the basidiocarpus has skeletal hyphae interspersed (Gilbertson 1981). Hibbet and Thorn (1994) reported the capture of the free-living nematode Rhabditis sp. by P. tuberregium. Cultivated in water-agar, the fungal mycelia produced toxin droplets, and when the nematodes came into contact with the toxin droplets, they became paralyzed and then colonized by the fungal hyphae. This mode of nematode capture has been previously demonstrated only in Pleurotus species genus, sensu stricto.
Regarding the nematicidal activity of the genus Pleurotus, several studies have been carried out on different stages of the sheep parasite nematode H. contortus, including the eggs, infectious larva (L3) and histotrophic larva (L4) (Aguilar-Marcelino et al. 2017b). Additionally, a biodirected chemical study has been reported by González-Cortazar et al. (2020); an initial chromatographic fractionation of the hydroalcoholic extracts of the fruiting bodies of P. djamor was carried out to identify and isolate the metabolites responsible for its nematicidal activity. The PdR2 fraction and the PdB subfraction obtained from the extract fractionation were evaluated in vitro against the nematode H. contortus. Additional evaluations were carried out in a gerbil model to determine the nematicidal effects of the PdB fraction. Finally, gasmass chromatography analyses, including spectrometry and nuclear magnetic resonance, of the PdB fraction were performed. The results showed that 100 % egg hatching occurred with 5 mg/mL of the PdB fraction. The larvicidal activity was > 97.2 % after 24 h with 20 mg/mL. The in vivo evaluation of the PdB fraction showed a 92.56 % reduction in H. contortus larvae. The compounds present in this fraction were a mixture of “allitol” and an unidentified terpene at a proportion of 9:1.
Next, some compounds that have been isolated from different species of Pleurotus genus with activity against different taxonomic genera of parasitic and free-living nematodes are presented (Table 2).
Table 2 Isolated compounds from different species of Pleurotus genus
| Species | Nematicide compound | Concentration | Reference |
| P. ostreatus | Trans-2-decenedioic acid | 95 % | Kwok et al., 1992 |
| P. pulmonarius | p-anisaldehyde, p-anisyl alcohol | 75 % | Stadler et al. 1994 |
| P. ferulae | Cheimonophyllon E, 5-hydroxymethylfurancarbaldehyde | 50 % | Li et al., 2001, 2007 |
| P. pulmonarius | 1-(4-methoxyphenyl)-1,2-propanediol | 75 % | Koitabashi et al. 2004 |
| P. ostreatus | 2-hydroxy-(4´-methoxy)-propiophenone | 87-94 % | Koitabashi et al. 2004 |
| P. eryngii | S-coriolic acid | 95 % | Xiang and Feng 2001 |
Pleurotus ostreatus
Known as white ears and oyster mushrooms, Pleurotus ostreatus has a cap in the shape of a shelf, is 4-14 cm in diameter, and whitish, gray or grayish-brown in color. Its gills are decurrent and whitish, and it has a short side stipe, which can sometimes be eccentric. The meat or context of the mushroom is white or whitish, with a pleasant taste and smell. The fruiting bodies grow in a gregarious manner and are generally overlapping on fallen or standing trunks, or on various plant remains. This fungus is distributed across the world in temperate and tropical forests (Salmones and Mata 2012).
The species P. ostreatus represents an alternative for parasite control in the livestock sector, as it has nematicidal activity against different taxonomic genera of nematodes that infect livestock (e.g., the eggs and infectious and histotrophic larvae of H. contortus), parasite nematodes of public health importance (e.g., Ancylostoma caninum) and free-living nematodes (e.g., Panagrellus redivivus) (Garcia-Lopes et al. 2015, Sanchez and Royse 2017).
The mechanism of action of edible mushrooms, particularly P. ostreatus, is carried out by structures known as “toxocysts” (Armas-Tizapantzi et al. 2019), which produce a nematotoxin called NRRL 3526 that acts against the bacteriophage nematode P. redivivus. At a concentration of 300 ppm, this toxin can induce 95% lethality in these nematodes in one hour (Kwok et al. 1992). The fungal mycelia invade the body orifices, such as the anus and the mouth of the nematode, feeding on the body to obtain nutrients and continue their biological cycle. Trans-2-decenedioic acid is produced by P. ostreatus and is among the secondary metabolites responsible (Kwok et al. 1992).
Several studies have reported up to 84 metabolites isolated from P. ostreatus with nematicidal activity (Degenkolb and Vilcinskas 2016, Kwok et al. 1992). In addition, other compounds with nematicidal activity have been identified, such as alkaloids, quinones, peptides, terpenoids, fatty acids (e.g., pentadecanoic acid, palmitic acid, sitosterol and linoleic acid), and polyphenols (Li and Zhang 2014, Pineda-Alegría et al. 2020). However, other species of fungi, such as Neolentinus ponderosus and Lentinula edodes (Montañez-Palma 2020), also have great potential in the search for bioactive products with nematicidal activity in the livestock arena.
Pleurotus ostreatus has been reported to produce a nematotoxin that can immobilize nematodes in a few minutes; however, the mechanism of action by which this paralysis occurs had not been established for this fungus-nematode interaction. Additionally, other studies have investigated the predatory relationship between free-living nematodes (Diplogastridae) and P. ostreatus as a potential method of exterminating other parasitic nematodes of plants, animals and public health importance. After nematode invasion, the fungus P. ostreatus defends itself by causing the nematode’s head to shrink. This phenomenon suggests that this antinematode mechanism is associated with linoleic acid peroxide (Satou et al. 2008).
Lee et al. (2020) conducted a study showing that P. ostreatus paralyses the free-living nematode Caenorhabditis elegans through a mechanism that is evolutionarily conserved in different nematode species. They found that the sensory “cilia” of C. elegans triggered a massive influx of intracellular calcium and a hypercontraction of the muscles in the pharyngeal wall and body, which eventually induced necrosis of the neuromuscular system throughout the nematode’s body.
Secondary metabolites of the genus Pleurotus and their activity against gastrointestinal nematodes
To date, various compounds with nematicidal activity have been isolated from fungal hyphae, including alkaloids, quinones, peptides, terpenoids, fatty acids, and polyphenols (Li and Shah 2014). This activity against nematodes is of great importance from the agricultural and livestock point of view, as well as in the biocontrol of pests since their action against phytopathogenic insects and fungi has also been mentioned. These characteristics make them excellent candidates as natural biocontrol agents.
The secondary metabolites responsible for this action include trans-2-decenedioic acid, produced by P. ostreatus, and s-coriolic acid, linoleic acid, p-anisaldehy-de, p-anisyl alcohol. However, the importance of these metabolites is questionable, as it has been shown that they are produced only in specific areas of the hyphae, and morphological differentiation to form specialized structures, called toxocysts, has only been shown in a few cases (Armas-Tizapantzi et al. 2019).
Several studies have reported the anthelmintic activity of Pleurotus species. A work carried out by Cuevas-Padilla et al. (2018) demonstrated the in vitro effects of a bioactive fraction from Pleurotus species. This bioactive fraction was evaluated against several stages of H. contortus, and it was shown that P. ostreatus had a higher nematicidal activity than P. eryngii. Later, Cuevas-Padilla (2019) carried out an in vitro evaluation of the proteins in raw extracts of five Pleurotus species (P. cornucopiae, P. djamor, P. eryngii, P. ostreatus and P. pulmonarius). The highest activity was obtained with P. pulmonarius, which was 81.2 % lethal against H. contortus eggs. They also revealed lethal effects against L1, L3 and L4, as well as mobility inhibition.
Comparisons between the different types of activities (i.e., paralysis by the raw extract, inactivation by the extracts, and a hydroalcoholic extracts) suggested the involvement of products of a proteinaceous nature. These results demonstrated the nematicidal potential of putative Pleurotus species. proteins against H. contortus larvae. Arteaga-Paredes (2018) demonstrated the nematicidal and nematostatic potential of P. ostreatus in vitro against Globodera pallida larvae (J2), a phytopathogenic nematode that generates losses of up to 30 % in potato crop yields (Solanum tuberosum) in Ecuador.
The nematicidal and nematostatic activity of the filtered broth of P. ostreatus was studied over time (8, 12 and 24 h) and at four different concentrations (0, 50, 75 and 100 %). The results showed that the percent mortality was directly proportional to the filtrate concentration and the exposure time. The most effective combination was a concentration of 100 % for 24 h, which resulted in a mortality rate of 41.6 %; treatment with a concentration of 100 % for 8 h resulted in the highest percent of immobilized nematodes (65.2 %). In another study (Aldaz 2018) it was reported that the fungus P. ostreatus has nematicidal activity. They initially obtained an extract using two solvents, acetone and methanol, through a Soxhlet extraction method. The nematodes used in these bioassays were Ditylenchus dipsaci and Panagrellus redivivus.
The acetone extract at a concentration of 75 % induced 95 % mortality in D. dipsaci and 92.55 % mortality in P. redivivus. Another study reported by Pineda-Alegría et al. (2017) evaluated the in vitro activity of extracts and fractions of P. djamor against H. contortus eggs and L3 larvae. The results showed that most of the P. djamor extracts had no significant activity against the eggs; however, one extract induced 77-98.7 % mortality in L3 larvae at 72 h post confrontation. Elucidation of the extract revealed several compounds including four fatty acids (pentadecanoic, hexadecenoic, octa-decadienoic, and octadecanoic acid, and a terpene identified as β-sitosterol).
Conclusions
Edible mushrooms of the genus Pleurotus produce secondary metabolites with anthelmintic activity against gastrointestinal parasites in their larval stages. There is excellent potential in this fungal genus for biotechnological applications due to its biocontrol characteristics. There are new lines of research that should be addressed to demonstrate its effectiveness in the livestock arena, since metabolites and other compounds with antihelminthic activity have been found in the mycelium, basidiomata, and degraded substrates where Pleurotus species had been cultivated. Among these potential investigations, the molecular approach stands out, as it is expected to help answer several questions, such as the mode of action and the conditions that predispose the fungus-nematode relationship.











 nueva página del texto (beta)
nueva página del texto (beta)