Introduction
One of the most worrying environmental problems concerns to soil contamination due to accidental oil spills and oil byproducts, which significantly contribute on loss of biodiversity in both agro- and ecosystems (Das and Chandran, 2011; Arellano et al., 2017). Thus, soil properties can be affected due to the exposure to petroleum hydrocarbons that limit the entry of oxygen and water (Pilon-Smits, 2005; Wang et al., 2013) and therefore, nutrient availability (Merkl et al., 2005; Bhalerao, 2013). Consequently, plants suffer an osmotic stress, affecting cellular biochemical processes (Xiong and Zhu, 2002; Galindo et al., 2017) largely because petroleum hydrocarbons have hydrophobic and lipophylic properties which significantly reduce water availability and root gas exchange (Robertson et al., 2007; Shukry et al., 2013).
Some biotic and abiotic factors may cause oxidative stress due to the production and accumulation of reactive oxygen species (ROS) such as singlet oxygen (1O2), superoxide anion (O2-), hydroxyl radical (OH-), and hydrogen peroxide (H2O2), which are generated during chemical reactions in cell compartments (Scandalios, 2005; Gill and Tuteja, 2010). Thus, ROS inhibit enzyme activities, and cause irreversible damages to proteins, nucleic acids and cell lipids; furthermore, the high accumulation of ROS may cause the death of plant cells (Ahmad et al., 2012; Lenoir et al., 2016a).
Soil contaminants [polycyclic aromatic hydrocarbons (PAH), diesel, and other fractions of petroleum hydrocarbons, for instance] may exacerbate the oxidative stress in plants whose balance of ROS production and scavenging mechanisms are impaired (Foyer and Noctor, 2005; Peralta-Pérez and Volke-Sepúlveda, 2012). Although ROS may potentially damage plant cells, they also play significant role as signaling compounds for controlling cell damage and death, allowing the plant adaptation to stressful conditions (Neill et al., 2002a, b; Bhattacharjee, 2005; Foyer and Noctor, 2005; Liu et al., 2009; Gill and Tuteja, 2010). Organic contaminants may cause toxic effects on plants which is related to the induction of oxidative stress (Peralta-Pérez and Volke-Sepúlveda, 2012; Lenoir et al., 2016a; Arias-Trinidad et al., 2017). For instance, Alkio et al. (2005) and Liu et al. (2009) showed that Arabidopsis thaliana under PAH-contamination had high production of ROS. Similar responses were described for plants of Nicotiana tabacum L. and Melilotus albus Medik., when grown under phenol and diesel contaminated substrates, respectively (Ibáñez et al., 2011; Hernández-Ortega et al.. 2012). However, the effects of petroleum hydrocarbons on the production of hydrogen peroxide and on the peroxidase activity in plants colonized with arbuscular mycorrhizal fungi (AMF) are not well understood.
The inoculation of AMF improves tolerance and the adaptation of plants under different stressful environments including petroleum hydrocarbon contaminated soils (Caravaca et al., 2005; Cho et al., 2006; Sheng et al., 2008; Xun et al., 2015), and this improvement is related to the growth promotion, the stimulation of microbial activity in the rhizosphere and the induction of antioxidant compounds or the release of enzymes by plants that degrade petroleum hydrocarbons (Joner and Leyval, 2003; Corgié et al., 2006). Moreover, AMF also improve phytoremediation of soils contaminated with petroleum hydrocarbons (Corgié et al., 2006; Alarcón et al., 2008; Hernández-Ortega et al., 2012; Xun et al., 2015). Although the inoculation and the benefits of AMF have been successfully tested on several horticultural, ornamental and tree species, few studies have been focused on understanding the role of AMF in plants that are established in petroleum contaminated soils (Alarcón et al., 2008; Zhou et al., 2013; Xun et al., 2015). Thus, this study evaluated the effects of AMF on the nutrient status, and on the hydrogen peroxide content and the peroxidase activity of leaves of Melilotus albus grown under diesel-contaminated sand.
Materials and methods
Experimental conditions
Autoclaved river sand was used as substrate for seed germination of Melilotus albus Medik, under green-house conditions (25-28 °C, 70 % relative humidity, 10 h light/14 h darkness). Thirty-six pots were inoculated with the AMF consortium Glomus Zac-19 (AMF) conformed by three species: Glomus claroideum, G. diaphanum, and G. albidum (Chamizo et al., 1998), and reclassified as Claroideoglomus claroideum [(Schenck & Sm.; emendation Walker & Vestberg) Walker & Schuessler], Rhizophagus diaphanus [(Morton & Walker) Walker & Schuessler], and G. albidum Walker & Rhodes (Schüßler and Walker, 2010; Redecker et al., 2013). This AMF consortium has been successfully evaluated as growth promoter of several plant species under different stressful conditions (Estrada-Luna and Davies, 2003; Cartmill et al., 2008). Another 36 pots were kept without AMF-inoculation as control plants. All pots were daily watered with distilled water and weekly fertilized with Long Ashton Nutrient Solution (LANS; Hewitt, 1966) modified to supply 11 μg P mL-1 to all treatments, to avoid interferences of this nutrient with the AMF-establishment.
Autoclaved sand was artificially spiked with diesel at 7500 mg kg-1. Once seedlings were colonized by AMF (after 40 days, with 50 % of mycorrhizal colonization) they were transplanted to either 270 g of contaminated or uncontaminated sand placed in containers of 300 g of capacity. Another set of non-inoculated plants (non-AMF) were also transplanted to either contaminated or no-contaminated sand. Plants were weekly fertilized with 50 mL of LANS as previously described, and watered as needed with distilled water.
Plant biomass accumulation and nutritional análisis
Thirty-five days after transplanting, plants were harvested to determine the dry weight (DW) of roots and shoots. Plant organs were dried at 70 °C for 72 h, and then, weighed with an analytical scale. The leaf elemental analysis (total nitrogen, phosphorus, calcium, magnesium, potassium, iron, copper, zinc and manganese) was performed via inductively coupled plasma mass spectrometry (ICP-AES Liberty Series II, EL97053010). The elemental analysis was performed by standardized protocols at the Laboratory of Plant Nutrition (Colegio de Postgraduados) based on Jones et al. (1991).
Leaf protein content, peroxidase activity, hydrogen peroxide content, mycorrhizal colonization, and total petroleum hydrocarbons (TPH) dissipation
Plant physiological parameters were also evaluated by taking leaf samples to measure the protein content, peroxidase activity (POX) and the hydrogen peroxide content. For the peroxidase activity, the plant tissue was macerated with liquid nitrogen by using the buffer (1:4 w/v) consisted on potassium phosphate 50 mM (pH 7.2), 5 mM DTT, 1 mM EDTA and 1 % polyvinyl-polypyrrolidone (Anderson et al., 1995). The soluble protein was isolated by centrifugation (10000 g) for 15 min, at 4 °C. The supernatant (extract) was utilized for measuring the protein (absorbance at 595 nm) and the enzymatic activity (POX) in a spectrophotometer AF-B0912H (HACH, USA). The POX was assessed as described in Srivastava and Dwivedi (1998), using guaiacol as substrate. The total reaction mixture (3 mL) consisted of 50 mM sodium phosphate buffer (pH 7.0), 3.33 mM guaiacol and 4 mM H2O2; the reaction was initiated by the addition of extracts (20 μL) at 26 °C. Progress of the reaction was directly measured by the increment in absorbance at 470 nm (ε =26.6/mM/cm) at 30 s intervals for 3 min at 26 °C. The protein quantification was determined by the Bradford method (Bradford, 1976), using bovine serum albumin (BSA) as standard.
For determining the hydrogen peroxide content, leaf tissue samples (0.2 g) were frozen in liquid nitrogen. Hydrogen peroxide was extracted in 1.2 mL of ice-cold 5 % (w/v) trichloroacetic acid (TCA) and centrifuged (10 min at 11000 g). Samples (0.5 mL) of the supernatant fraction were passed through a Dowex-1 resin (0.5 g, Sigma® D-2928) column followed with 3.5 mL of 5 % TCA. The H2O2 was measured in the eluates using the luminol-dependent chemiluminescence method of Warm and Laties (1982): 0.5 mL of eluate were added to 0.5 mL of 0.5 mM luminol, the volume was adjusted to 5 mL with 0.2 M NH4OH (pH 9) in a borosilicate glass tube was analyzed by using an Optocomp P luminometer (MGM Instruments, USA). Chemiluminescence was initiated by injecting 50 μL of 0.5 mM potassium ferricyanide in 0.2 M NH4OH and emitted photons were counted over 5 s. A parallel sample of each initial extract was processed after addition of a known concentration of H2O2 to provide a recovery correction factor (Warm and Laties, 1982).
After 35 days of transplanting to diesel contaminated sand, the mycorrhizal colonization was estimated via the alkaline phosphatase vital stain procedure (Pearse, 1968; Tisserant et al., 1993) at 10 sampling times, in which three plants were analyzed. In brief, roots were incubated for two hours at room temperature in a digester solution consisting of 0.05 M Tris/citric acid buffer (pH 9.2), 0.05 % sorbitol, 15 units cellulose mL-1 and 15 units pectinase mL-1. Roots were exposed to a sodium chloride solution (1 % active chlorine) for five minutes. Staining procedure consisted on overnight exposure of roots to reaction medium consisted on 0.05 Tris/citric acid buffer (pH 9.2), 1 mg mL-1 fast blue RR salt, 1 mg mL-1 α-naphtyl acid phosphate, 0.5 mg mL-1 MgCl2 and 0.8 mg mL-1 MnCl2·4H2O. Fractional colonization was estimated microscopically as the intensity of AMF-colonization of the root cortex, expressed as a percentage. In addition, AMF colonization was also estimated by clearing roots and staining as indicated in the procedure of Phillips and Hayman (1970), to identify the typical fungal structures in roots.
After 35 days of transplanting, the analysis of TPH from diesel-contaminated sand was performed by a modified EPA SW-846 Method 8270B (Louchouarn et al., 2000; USEPA, 1986). Extractions were performed using 100 % hexane and the extracts were used for the quantitative determination of TPH by gas chromatographic mass spectrometry (GC-MS, Agilent Technologies, model 6890N, Net Work GC system). Samples from AMF-plants and non-AMF plants were collected and analyzed by comparing their TPH-mass spectra (HP Chemstation-NIST 05 Mass spectral search program, version 2.0d) to that obtained from initial samples (time zero) collected from sand after 24 h of diesel contamination to estimate the percentage of TPH degradation.
Experimental design and statistical analysis
A 2×2 factorial experiment was conducted with four treatments and 12 replicates, distributed in a completely randomized design. Factors were as follows: two levels of diesel (0 and 7500 mg kg-1) and two levels of AMF-inoculation (non-AMF and AMF plants). Normality of data was checked out by using the test Shapiro-Wilks (P < 0.05). The mean comparison test of t-student was utilized for comparing results of TPH-degradation (n= 6), while data from mycorrhizal colonization (n=9) were analyzed by the mean comparison test of Wilcoxon. Data from plant biomass (n= 5) and nutrition (n= 3), as well as soluble protein content (n= 6), POX activity (n= 4) and content of H2O2 (n= 3) were analyzed by the Kruskal Wallis test (α= 0.05). The analyses were performed by using InfoStat (InfoStat, 2008).
Results
Plant biomass accumulation and nutritional analysis
The AMF inoculation as independent factor had no significant effects on plant biomass accumulation, but under uncontaminated sand, AMF plants had significantly low shoot DW (Figure 1B). In contrast, diesel factor significantly (p≤0.01) impaired plant growth, as seen in Figure 1A and B. Total DW of either roots or shoots was reduced in plants under diesel contamination; however, non-significant effects were observed between AMF plants and non-AMF plants (Figure 1B).
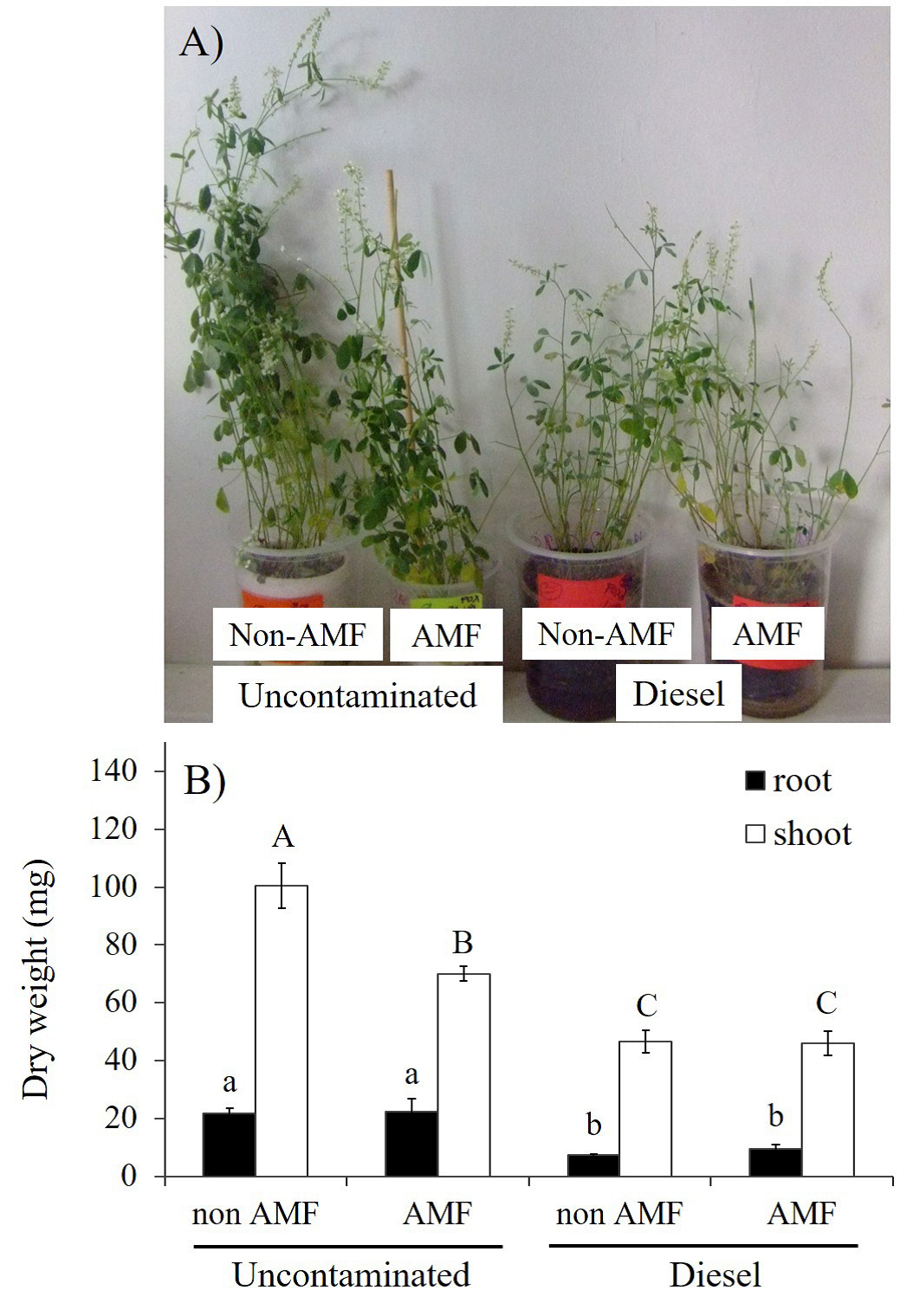
Figure 1 Effects of arbuscular mycorrhizal fungi (AMF) and diesel contamination (7,500 mg kg-1) of Melilotus albus, after 35 days. A: Plant growth responses. B: Dry weight of roots and shoots. Means ± Standard error. n=5. Bars with different letters differ statistically from each other (Kruskal-Wallis, α = 0.05).
Although no significant effects were detected, AMF-inoculation resulted in lower reduction of the total content of either macro or microelements (Table 1). In contrast, diesel contamination resulted in significant reductions on N, P, K, and Ca (Table 1); however, plants under diesel contamination had significant increase in the total content of Fe (Table 1). In regards to the AM-F×Diesel interaction, the treatment non-AMF + 7500 mg kg-1 of diesel resulted in significantly increased total content of Fe (Table 1). The significant highest values of N, P, and K were achieved in the treatment non-AMF without diesel (Table 1).
Table 1 Total element content in shoots of Melilotus albus with or without inoculation of the arbuscular mycorrhizal fungal consortium Zac-19 (AMF), under diesel-contaminated sand (7,500 mg kg-1), after 35 days after transplanting
| Factors/Levels | N | P | K | Ca | Mg | Fe | Cu | Zn | Mn |
| g plant-1 | mg plant-1 | ||||||||
| Mycorrhiza | |||||||||
| non-AMF | 1.04±0.18 a§ | 0.12±0.03 a | 0.97±0.16 a | 0.38±0.04 a | 0.21±0.01 a | 14.76±4.18 a | 0.09±0.04 a | 1.42±0.34 a | 8.34±1.5 a |
| AMF | 0.82±0.12 a | 0.08±0.01 a | 0.70±0.1 a | 0.37±0.04 a | 0.23±0.02 a | 8.94±1.37 a | 0.07±0.04 a | 0.94±0.21 a | 4.46±0.7 a |
| Diesel (mg kg-1) | |||||||||
| 0 | 1.24±0.09 a | 0.14±0.02 a | 1.12±0.1 a | 0.44±0.04 a | 0.24±0.02 a | 7.51±0.89 b | 0.09±0.03 a | 1.32±0.39 a | 4.49±0.65 a |
| 7500 | 0.62±0.04 b | 0.06±0.004 b | 0.55±0.04 b | 0.31±0.03 b | 0.20±0.02 a | 16.19±3.8 a | 0.06±0.02 a | 1.03±0.14 a | 8.31±1.5 a |
| Mycorrhiza x Diesel | |||||||||
| non-AMF + 0 | 1.41±0.13 a | 0.18±0.02 a | 1.32±0.06 a | 0.46±0.05 a | 0.23±0.02 a | 7.64±0.41 b | 0.12±0.04 a | 1.92±0.52 a | 5.34±0.78 a |
| AMF+0 | 1.08±0.01 ab | 0.01±0.01 ab | 0.92±0.06 ab | 0.42±0.06 a | 0.26±0.03 a | 7.38±1.96 b | 0.06±0.02 a | 0.73±0.37 a | 3.63±0.90 a |
| non-AMF + 7500 | 0.67±0.06 b | 0.06±0.01 b | 0.61±0.06 b | 0.30±0.03 a | 0.20±0.02 a | 21.89±6.04 a | 0.02±0.0 a | 0.92±0.21 a | 11.33±1.33 a |
| AMF + 7500 | 0.57±0.06 b | 0.06±0.01 b | 0.48±0.05 b | 0.33±0.06 a | 0.20±0.03 a | 10.49±1.76 ab | 0.07±0.03 a | 1.15±0.22 a | 5.28±0.99 a |
| AMF | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Diesel | 0.01 | 0.01 | 0.01 | 0.05 | NS | 0.01 | NS | NS | NS |
| AMFxDiesel | 0.05 | 0.05 | 0.05 | NS | NS | 0.05 | NS | NS | NS |
§Means with the same letter in each column for each individual factor and corresponding levels, are not statistically different (Kruskal Wallis, α=0.05); NS= no significant, n=3.
Leaf protein content, peroxidase activity, hydrogen peroxide content, mycorrhizal colonization, and TPH degradation
Leaf protein content was significantly affected (p≤0.01) by AMF condition as independent factor; thus, mycorrhizal inoculation resulted in 13.8 % lower protein content than non-AMF plants (Table 2). In contrast, diesel application did not significantly affect the protein content, but plants exposed to diesel showed a 9.5 % reduction of the protein content when compared to plants without diesel (Table 2). The interaction of both factors had significant effects (AMF×Diesel, p≤0.01) on the leaf protein content. Under uncontaminated sand, non-AMF plants had significantly greater protein content than AMF plants (Figure 2A), but at diesel contaminated sand either non-AMF or AMF plants showed similar leaf protein content (Figure 2A). Overall, under diesel contamination, non-AMF plants showed a significant reduction (18.2 %) in the protein content when compared to non-AMF plants grown at uncontaminated sand (Figure 2A).
Table 2 Protein content, peroxidase activity, and peroxide content in leaves of Melilotus albus with or without inoculation of the arbuscular mycorrhizal fungal consortium Zac-19 (AMF), under diesel-contaminated sand (7,500 mg kg-1), after 35 days after transplanting
| Independent factor and levels | Protein content | Peroxide content | Peroxide content |
| (mg g-1) | (U mg-1 of protein) | (µM g-1) | |
| Mycorrhizal | |||
| non-AMF | 39.11 ± 1.28 a § | 1.22 ± 0.26 b | 9.36 ± 1.26 a |
| AMF | 33.68 ± 0.31 b | 4.35 ± 0.45 a | 8.74 ± 1.68 a |
| Diesel (mg kg-1) | |||
| 0 | 38.22 ± 1.50 a | 2.13 ± 0.62 a | 5.77 ± 0.39 b |
| 7500 | 34.57 ± 0.47 a | 3.44 ± 0.58 a | 12.32 ±0.16 a |
| AMF | 0.01 | 0.01 | NS |
| Diesel | NS | NS | 0.01 |
| AMF x Diesel | 0.01 | 0.01 | 0.05 |
§Means with the same letter in each column for each individual factor, are not statistically different (Kruskal Wallis, α=0.05). NS= no significant, n=8.
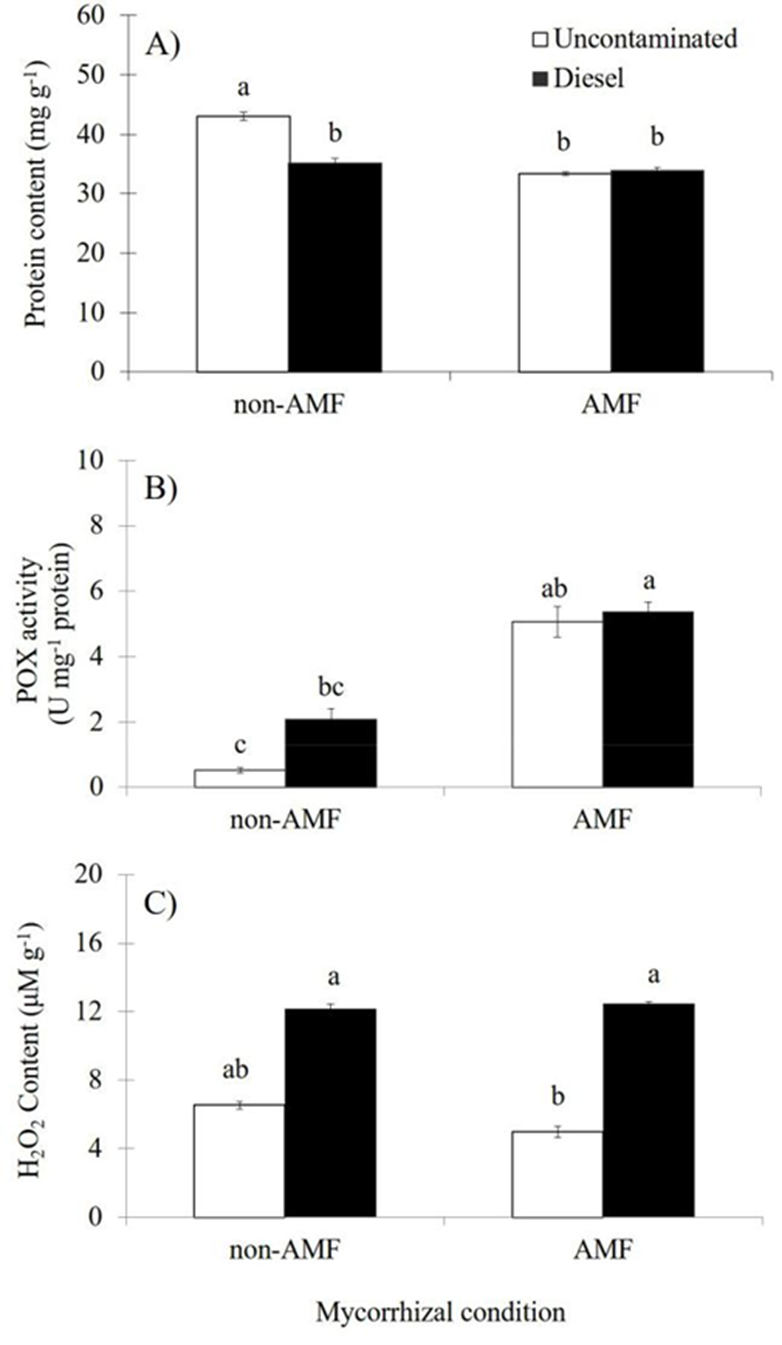
Figure 2 Effects of arbuscular mycorrhizal fungi (AMF) and diesel contamination (7,500 mg kg-1) in leaves of Melilotus albus, after 35 days. A: Protein content. B: Peroxidase activity. C: Hydrogen peroxide content. U = enzymatic units (nmol min-1). Means ± Standard error. n=4. Bars with different letters differ statistically from each other (Kruskal Wallis, α=0.05).
The POX activity was significantly affected by AMF as independent factor (p≤0.01); AMF plants had higher (3.5 fold) POX activity than non-AMF plants (Table 2). In contrast, plants under diesel contamination did not have significant effects on this variable, but plants grown at diesel contaminated sand had 61 % more POX activity than plants without diesel (Table 2). The interaction of both factors had significant effects (AM-F×Diesel, p≤0.01) on the POX activity. Either at uncontaminated or at contaminated sand, AMF plants had significantly greater POX than non-AMF plants (Figure 2B). Overall, AMF plants regardless diesel contamination showed increased POX activity than non-AMF plants (Figure 2B).
The content of peroxide in leaves was not significantly affected by AMF as independent factor; however, AMF inoculation resulted in 6.6 % reduced peroxide content than non-AMF plants (Table 2). Diesel contamination caused significant effects (p≤0.01) on the peroxide content; plants under diesel contamination had higher (2.1 fold) peroxide content than plants without diesel (Table 2). The interaction of both factors had significant effects (AMF×Diesel, p≤0.05) on the peroxide content. Overall, under uncontaminated sand, AMF plants had lower peroxide content than non-AMF plants (Figure 2C); in contrast, at contaminated sand either non-AMF or AMF plants showed similar peroxide content (Figure 2C). Regardless AMF inoculation, diesel contamination resulted in significantly increased peroxide content in leaves (Figure 2C).
Mycorrhizal colonization determined by either ALP staining or trypan blue staining was reduced (p≤0.01) due to contamination (Figure 3). In the absence of diesel, the total AMF colonization detected by trypan blue staining was 41.8 %; from this percentage, about 72% of it, is responsible for transferring P to the host as detected by the ALP staining procedure. In contrast, under diesel contamination, only 37 % of the total AMF colonization may account for the P-transfer to the host (Figure 3). On the other hand, diesel caused significant decrease (p≤0.01 or p≤0.05) on the proliferation of hyphae (>46 %), vesicles (>45 %), and arbuscules (>84 %) (Table 3), as measured by the classical root staining procedure of Phillips and Hayman (1970).
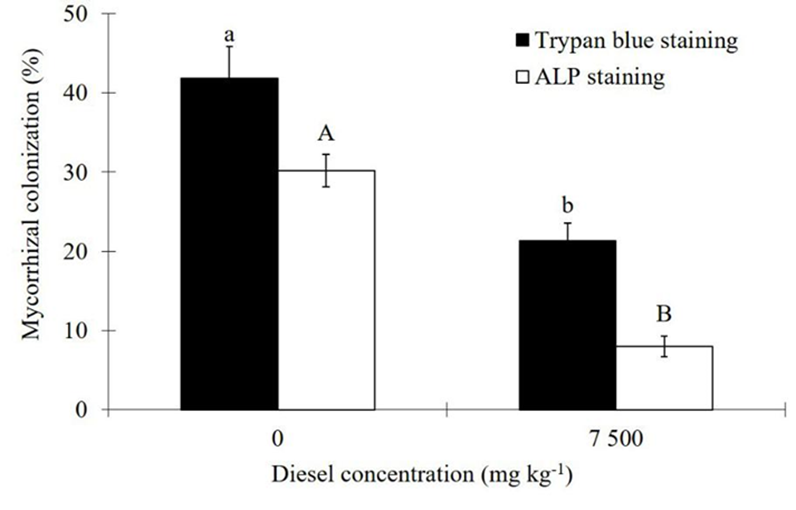
Figure 3 Effect of diesel contamination (7,500 mg kg-1) on the colonization of arbuscular mycorrhizal fungi (AMF) in Melilotus albus, measured by the alkaline phosphatase (ALP) vital stain and by the trypan blue staining, after 35 days. Means ± Standard error. n=9. Bars with different letters differ statistically from each other (Wilcoxon for independent samples, α=0.05).
Table 3 Arbuscular mycorrhizal colonization in roots of Melilotus albus determined by clearing and staining roots with trypan blue, after 35 days of after transplanting to diesel contaminated sand
| AMF | Diesel | Hyphae | Vesicles | Arbuscules |
| Condition | mg kg-1 | % | ||
| AMF | 0 | 41.61 ± 3.94 a § | 13.64 ± 1.86 a | 20.05 ± 2.83 a |
| AMF | 7 500 | 19.79 ± 1.95 b | 6.64 ± 0.74 b | 2.40 ± 0.53 b |
|
|
|
|
|
|
| P-value | 0.01 | 0.01 | 0.001 |
§Means with the same letter in each column are not statistically different (Wilcoxon test for independent samples). n=9.
The TPH dissipation from contaminated sand was significantly enhanced (p<0.01) due to AMF-inoculation. Thus, AMF plants contributed to 46.8 % of TPH dissipation while non-AMF plants had a dissipation of 22.9 % (Figure 4).
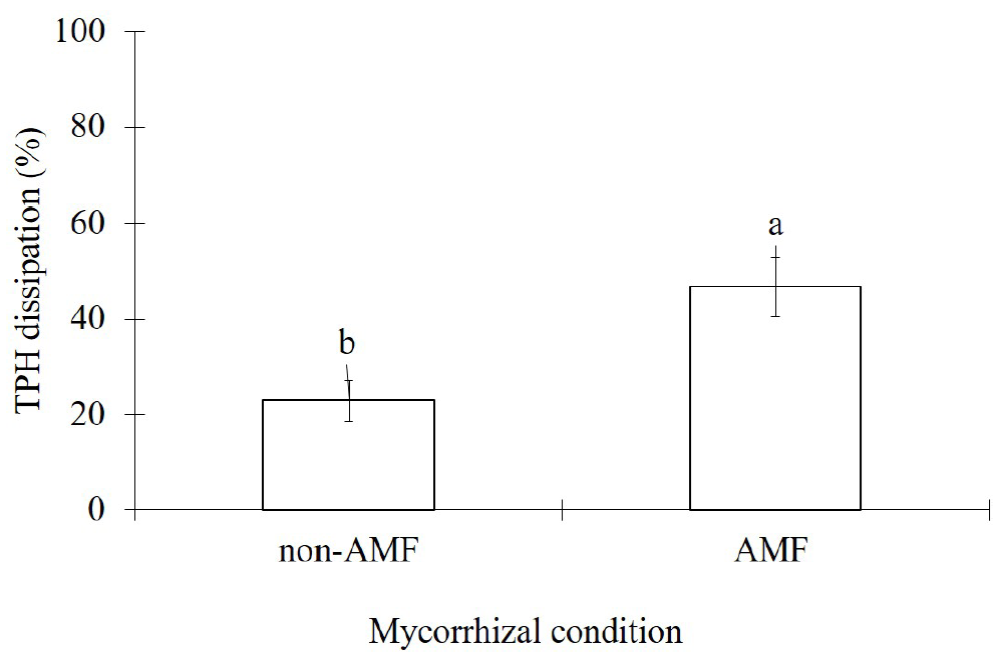
Figure 4 Effects of arbuscular mycorrhizal inoculation (AMF) in Melilotus albus on the total petroleum hydrocarbons dissipation from diesel contaminated sand (7,500 mg kg-1), after 35 days. Means ± Standard error, n=6. Bars with different letters differ statistically from each other (t-student, α=0.05).
Discussion
Diesel exposure caused negative effects on the growth of M. albus, which may be related to the limited water and nutrient uptake by roots (Quiñones-Aguilar et al., 2003; Sangabriel et al., 2006). No significant differences were observed on plant biomass accumulation between non-AMF plants and AMF-plants in the presence of diesel. The latter is opposite to those AMF benefits described for several plant species at contaminated soils (Joner and Leyval, 2003; Alarcón et al., 2008; García-Sánchez et al., 2014; Xun et al., 2015).
At uncontaminated sand, AMF inoculation resulted in lower plant biomass production when compared to non-AMF plants, which may be a result of growth limitation of plants grown in a small container, but same amount of substrate, which may have restricted both root expansion and growth, and fast nutrient depletion in sand. Nevertheless, the amount of sand (either contaminated or not) was the same (270 g) for each container. In this regard, plant growth may be dependent on container size (Ray and Sinclair, 1998; Bouzo and Favaro, 2015; Oagile et al., 2016). Moreover, Gallegos et al. (2020) indicate that the container shape did not exert significant effects on plant growth when using the same amount of substrate.
In addition, the AMF symbiosis may have exerted a strong sink of carbon sources for further fungal development in roots. In this regard, the AMF symbiosis in plants may demand between 20 and 30 % of C-derived from photosynthesis (Smith and Read, 1997), and this may explain in part the negative effects of AMF on biomass production of plants grown at uncontaminated sand, even though the nutrient solution was applied weekly.
There is not much available information about the contribution of AMF on the nutritional status of plants exposed to petroleum hydrocarbons. However, some reports have demonstrated that petroleum hydrocarbons may negatively affect the uptake and content of both macronutrients and micronutrients in Lolium multiflorum (Alarcón, 2006), as accounted in general, in this research for M. albus. The contribution of AMF on the uptake and translocation of macro- and microelements to plants has been previously demonstrated (Smith and Read, 1997); however, our data show that AMF inoculation did not favor the nutrient content of plants, regardless diesel contamination. One of the main benefits of AMF is related to improve P-uptake, but under our experimental conditions AMF plants resulted in lower P-content than non-AMF. The latter concurs with Kobae (2019) who indicated that the P-uptake in mycorrhizal plants is dependent on the fungal phenotype strain. In the same manner, the inoculation of Medicago sativa with Funneliformius mosseae resulted in lower P-content when compared to non-AMF plants (Mensah et al., 2015); this effect was also reported in soybean plants inoculated with Glomus aggregatum (Wang et al., 2016). By relating the negative effects of AMF on plant growth and nutrition under our experimental conditions, it seems that AMF did not have good compatibility with plants of M. albus; moreover, under diesel contaminated sand, plants harbor the AMF which act as sink for C-derived from photosynthesis, thus, allowing fungal survival under stressful conditions, and causing additional plant growth impairments. Furthermore, AMF under diesel contamination seem to colonize plant roots for protecting themselves from the soil contaminant, but without providing benefits on growth nor nutrient uptake, but inducing certain physiological benefits that alleviate plant stress due to toxic the effects of petroleum hydrocarbons. However, further research is needed to asses this hypothesis.
Diesel caused negative effects on the protein content of leaves of M. albus. Previous reports have indicated that petroleum hydrocarbons such as diesel or phenanthrene also had significant decrease on the protein content in plants like Zea mays L., Sorghum bicolor L. or Ocimum gratissimum L. (Tang et al., 2009; Dubrovskaya et al., 2014; Ekpo et al., 2014). Our results indicated that AMF maintain slightly low protein content in plants grown at either diesel contaminated or uncontaminated sand, and this response may account on maintaining “normal” protein metabolism in AMF plants grown at diesel contamination (Tang et al., 2009).
The present study also shows some physiological benefits of AMF in M. albus exposed to diesel stress. The POX was significantly enhanced due to diesel contamination, indicating that plants were subjected to a stressful condition, then, resulting in impaired plant growth. The stress induced by diesel resulted in increased production of H2O2 in M. albus, and this compound may cause detrimental effects on plant cells; thus, plants must trigger specific metabolic responses (for example, the synthesis and/or the activity of antioxidant enzymes or non-enzymatic compounds) for scavenging ROS accumulation (Mittler, 2002; Hao et al. 2005; Lee et al., 2007; Huang et al., 2008). The later may explain the high POX activity obtained for either non-AMF or AMF plants grown under diesel contamination. The AMF inoculation conferred more tolerance to plants grown in the presence of diesel, which may be related to the induction of antioxidant activities that are directly related to plant responses when facing petroleum hydrocarbons and other organic contaminants (Hernández-Ortega et al., 2012; García-Sánchez et al., 2014; Xun et al., 2015). The AMF inoculation significantly increases the activity of specific antioxidant enzymes or non-enzymatic antioxidants of plants exposed to abiotic stresses (Alguacil et al., 2006; He et al., 2007; Cartmill et al., 2008; Xun et al., 2015). Nevertheless, the physiological benefits of AMF on plants exposed to petroleum hydrocarbons have received little attention (Criquet et al., 2000; Alarcón et al., 2008).
In the same manner, the evaluation of AMF effects on the stimulation/inhibition of enzymatic or non-enzymatic compounds who are responsible for alleviating and detoxifying plants when growing in the presence of organic contaminants must be taken in consideration, in combination with specific micronutrients that may participate as cofactors for some antioxidant enzymes (Lee et al., 2007). In this regard, this may in part explain how AMF plants may have better physiological mechanisms to avoid potential oxidative stress when growing at diesel contaminated substrates.
Some antioxidant enzymes such as peroxidases may play an important role on alleviating toxicity in plants under diesel contamination. At uncontaminated sand, AMF showed decreased content of H2O2, indicating less oxidative conditions, but more favorable conditions to plant cells. In contrast, the presence of diesel resulted in similar content of H2O2 between AMF and non-AMF plants. Thus, these H2O2 levels may be related to cell signaling that may allow specific responses for inducing plant tolerance to diesel contamination. However, this hypothesis needs more research for being tested when plants with or without AMF are facing toxic effects of petroleum hydrocarbons.
AMF colonization in M. albus diminished due to diesel contamination; nevertheless, although the total AMF colonization was significantly reduced, approximately 37 % of it remains functional as demonstrated by the colonization estimated with the ALP staining, which suggests potential transfer of P to the host under diesel contamination. Some reports indicate the negative effects of petroleum hydrocarbons on AMF colonization (Rabie, 2004; Nwoko, 2014; Alejandro-Córdova et al., 2017); in contrast, the AMF colonization in Echinochloa polystachia H.B.K, detected via ALP staining, was not diminished by benzo[a]pyrene (Alarcón et al., 2006). AMF-colonization diminished along experimentation due to diesel toxicity; however, we suggest that this reduction may also be in part a result of plant phenology, since plant flowering was observed at 25 days after transplanting to the contaminated sand (data not showed). Thus, AMF may reduce their colonization as plant reproductive organs develop (for instance, flowers, fruits and seeds), which are strong sinks of photosynthesis-derived carbon, thus limiting the carbon-availability for AMF in cortical cells (Bago et al., 2003; Ijdo et al., 2010). The reduction of AMF colonization because of diesel contamination may limit plants to cope the stress induced by petroleum hydrocarbons as described by Hernández-Ortega et al. (2012).
Furthermore, AMF contribute to the degradation/dissipation of petroleum hydrocarbons in the rhizosphere (Volante et al., 2005; Alarcón et al., 2008; Cheung et al. 2008; Lenoir et al., 2016b). Our results provides information about the influence of AMF during the phytoremediation of diesel hydrocarbons. Thus, these fungi are important rhizosphere elements that favor the degradation and/or the detoxification of several fractions of petroleum hydrocarbons (Alarcón et al., 2008; Cheung et al., 2008; Wu et al., 2009; Iffis et al., 2017). However, further research is needed to evaluate the interaction between AMF and plants, as well as the potential combinations of AMF with beneficial hydro-carbonoclastic microorganisms for improving phytoremediation of contaminated soils.
Conclusions
Diesel contamination negatively affected both growth and nutritional status of M. albus. Conversely to scientific literature, the inoculation of the AMF-consortium Zac-19 did not improve the biomass production nor the nutrient status of plants under diesel contamination in comparison to non-AMF plants. Regardless AMF inoculation, diesel contamination enhanced both content of H2O2 and POX activity that may be associated to better plant fitness and adaptation to the negative effects of diesel. Moreover, AMF-inoculation significantly influenced the alleviation of the toxic effects of diesel on M. albus by keeping more balanced H2O2 accumulation and by enhancing the peroxidase activity in leaves. Furthermore, AMF contributed on improved TPH dissipation (>45 %) in the rhizosphere.











 nueva página del texto (beta)
nueva página del texto (beta)



