Introduction
Soil aluminum toxicity is considered a problem affecting 30-40 % of the world’s arable land, second only to drought, and both represent a major constrain to maximizing crop yields. Highly degraded soils, or those that developed in acid material, exhibit a low pH (<5), limiting the availability of base cations and raising the availability levels of aluminum (Kelly et al., 2005). There are biological methods for the recovery of soils contaminated by heavy metals and other contaminants including the use of plants and rhizospheric microorganisms, which represent a low-cost alternative with several environmental advantages (González-Chávez, 2005). Soil microorganisms play a key role in the mobilization and immobilization of metal cations, as well as in changes in their availability for plants.
Arbuscular mycorrhizal fungi (AMF) are soil microorganisms that establish a mutualistic symbiosis with most plants, providing a direct physical link between soil and plant roots. AMF are found in all habitats and climates, including disturbed soils (such as those derived from mine activities) or degraded soils (Batista and Sánchez, 2009). The AMF symbioses benefit plants growing in acidic soils, because they enable greater access to nutrients, improving their metabolism, and therefore promote a decrease in stress in host plants. This has been reported in heavily degraded tropical soils (Cardoso and Kuyper, 2006), deciduous forests (Yamato and Iwasaki, 2002; Postma et al., 2007; Diehl et al., 2008) and also in extremely acidic environments (Cumming and Ning, 2003; Maki et al., 2008; Taheri and Bever, 2010). The extensive external network of hyphae explores a large volume of soil and can reach scarce and immobile nutrient resources like P and Zn that would not otherwise be available to the roots. In addition, AMF can reduce the accumulation of metals and other elements, such as Fe and Mn, which are problematic in acidic soils (Cumming and Ning, 2003).
Plant growth in acidic soils is limited by a set of conditions, including excess protons (H+), aluminum, and some nutrients found in excessive amounts, such as manganese, or essential nutrient deficiencies such as phosphorus, calcium, magnesium and molybdenum (Driscoll et al., 2001; Bolan et al., 2003; Fageria and Baligar, 2008), that are essential for plants. Aluminum toxicity is a complex problem in agricultural soils, involving direct effects of Al on soil biota and indirect effects deriving from soil acidity and causing nutrient imbalances in plants which in turn affect soil biota. Aluminum is a non-essential trace element entering cells via transporters or channels for essential elements with similar physicochemical properties. Cellular damage is overcome by keeping low intracellular concentrations of these elements, by blocking them outside the hyphae, by increasing their extrusion or by detoxifying them once inside the fungal cell (Ruytinx et al., 2016). Plants exposed to Al show inhibition of cell division and root elongation, which is one of the most marked and initial symptoms of the toxicity of this metal and occurs within a few hours or even minutes after exposure (Giannakoula et al., 2010). Plants vary considerably in their tolerance to Al, but certain species can be affected already at 5 μM Al (Delhaize and Ryan, 1995; Matsumoto et al., 2001). Some soils having naturally high levels of Al and organisms living there may have high tolerance to Al (Osaki et al., 1997), whereas others are tolerant even without having experienced exposure to Al due to their genetic plasticity (Kochian et al., 2004). Therefore, toxicity concentrations and limits are difficult to establish and are not defined in the literature as they depend on the soil type and on the organism. AMF are involved in the remediation of Al toxicity. Several studies report an increase in Al resistance in root systems colonized by AMF (Cuenca et al., 2001). The immobilization of Al, and the different exclusion mechanisms active in the roots of plants colonized by AMF, can contribute to the resistance to stress acquired in the host plant. In these cases, Al may be extra-cellularly attached to the wall of the fungal cell or intra-cellularly retained by polyphosphate granules in fungal vacuoles (Toler et al., 2005; González-Guerrero et al., 2008; Zhang et al., 2009). In addition to this, AMF may precipitate non-essential metals in the soil. Some, but not all, AM fungi have been shown to alter organic acid exudation and to produce high amounts of citrate in the presence of Al (Klugh and Cumming, 2007). Fungi forming ericoid, ectomycorrhizal and arbuscular mycorrhizae differ in their strategies and mechanisms to cope with metal toxicity (Rutyinx et al., 2016) and although it has been shown that Ascomycetes and Basidiomycetes present evolutionary adaptation to metalliferous soils (Cumming et al., 2001; Colpaert et al., 2004), fungi living in recently polluted soils are likely not adapted and will be selected according to their ability to tolerate toxicity or escape from it. AMF species and isolates of the same species are known to differ in their capacity to induce Al tolerance in plants (Kelly et al., 2005), which suggests AMF should differ in their tolerance to Al as well. The question remains if AMF capable of tolerating and coping with Al toxicity belong to certain families or species, if some AMF can tolerate higher toxicity levels than others, or if AMF communities from soils exposed to metals have selected metal tolerant species. To date, most research has assumed AMF are tolerant to Al and have focused on the capacity of AMF to increase plant tolerance to Al toxicity. Also, most studies have exposed both plants and AMF to Al because AMF could not be cultured without a host and found some differences in tolerance among isolates of different families, genera and species but so far few isolates have been tested (reviewed by Seguel et al., 2013). Therefore, we still know very little about how toxic Al is to AMF independently from the host and how widespread is Al tolerance present in AMF. Moreover, Al pollution is associated to soil acidity, which also affects AMF and other soil biota development and functioning, and we ignore if Al affects AMF directly, through the host plant, or through the effects of soil acidification.
This study was conducted to explore direct toxicity effects of Al on AMF. To do so we used a compartmentalized system that allowed us to expose the external mycelium to increasing Al concentrations without affecting the host plant and without the interference of soil pH changes. We tested two AMF species without prior exposure to aluminum and a native AMF community from a soil with recent (decades) contamination with Al and hypothesized that the native AMF community from the Al-contaminated soil will show higher tolerance to Al than the two isolates without previous exposure to this metal. In other words, we hypothesized that prior exposure to Al pollution had selected Al tolerant species in the native community.
Materials and methods
Soil and biological materials
The soil used in the experiments was an Acrisol (WRB) extracted from a maize field within the city of Morelia, Michoacán, Mexico. The soil is a clay, with 2.74 % organic matter, 23.2 mg N and 5.8 mg P kg-1 soil, and pH of 6-6.5. It contains 1.18 mg kg-1 soil or 0.01 milliequivalents 100 g-1 soil of exchangeable aluminum, which can be considered high for soils not exposed to Al contamination or naturally rich in Al. However, <2 me 100 g-1 likely do not represent a problem for development of most crops given that the soil pH is only slightly acid and would not favor the release of Al3+ (Rivera-Méndez et al., 2016). A soil without Al contamination was chosen to be able to establish the desired levels of this metal by adding it gradually. However, it is known that increasing aluminum increases soil acidification, which in turn affects the function of AMF and its association with plant roots (Clark, 1997). In order not to confuse the effect of acidification with the toxicity effect of Al, pH tests were performed as the application of soil aluminum increased. The soil was mixed with sand to facilitate the incorporation of Al, but it was also observed that the addition of sand contributed to increase acidity by decreasing adsorption capacity to mineral particles. After several tests it was found that the ratio soil:sand 9:1 and the addition of 50 and 100 mg kg-1 of Al were the only combinations that allowed to maintain the pH to a maximum of 0.5 units of pH from the initial pH of the soil. A greater amount of Al could reduce the pH by up to two units. Taking these results into account it was considered that the aluminum concentrations to be applied in the experiment would be 0, 50, 100 mg Al kg-1 soil. The mixture of soil with sand in 9:1 ratio was autoclaved for 1 h at 120 oC, on two occasions, allowing aeration between each disinfection to avoid toxicity. For the application of aluminum, the soil mixture was extended on trays and sprayed evenly with solutions containing the amount of aluminum chloride needed for the amount of soil and dissolved in deionized water. The soil was gradually moistened with an atomizer, stirring continuously, and allowed to dry in the sun to allow adsorption of aluminum. The control treatment was sprayed with water and processed in the same way as Al treatments.
Three AMF inoculation treatments were chosen for the experiment: the native AMF community from a soil contaminated with Al, two AMF isolates from soil not contaminated with Al, Acaulospora delicata C. Walker, C.M. Pfeiff. & Bloss and Gigaspora margarita W.N. Becker & I.R. Hall, and a control treatment without AMF. The native community inoculum from Al contaminated soil was collected from an agricultural plot with chickpea and maize cultivation close to the city of León, Guanajuato, where insufficient regulation of waste-water treatment from footwear factories, leather crafts and leatherworking, makes them a source of constant contamination to the surrounding irrigated soils. This soil is a dark gray vertisol containing 120 mg Al kg-1 soil, two orders of magnitude more exchangeable Al than the soil used for the soil:sand substrate of the experiment. The inoculants of Gigaspora margarita and Acaulospora delicata were obtained from a soil collected in a maize field in Tiripetío, Michoacán, Mexico, a region with Luvisol soil without aluminum contamination by the method of wet sieving and decanting proposed by Gerdemann and Nicolson (1963). These species were selected because they had abundant AMF spores in good condition. Once the spores were identified and separated by their morphology, they were propagated by the trap plant culture method (Carreón-Abud et al., 2013). Grass seeds were disinfected and sown in pots with autoclaved soil. The successful trap cultures from Tiripetío and the soil with native community of AMF from León were multiplied in greenhouse by adding sterile soil and new grass host plants to the trap cultures or field soil. After 8 weeks, the soil was left to dry for a week, the grass shoots were cut, and the grass roots were chopped and mixed thoroughly with the soil.
Experimental set-up
The experiment was carried out in a completely randomized factorial design with two factors: 1) aluminum added to the soil (0, 50 and 100 mg kg-1) and 2) four levels of AMF inoculation (Acaulospora delicata, Gigaspora margarita, the native community of an aluminum-contaminated soil, and a control without AMF). Each of the 12 treatments had five replicates.
A growth system was designed with two compartments (Figure 1), which consisted of PVC tubes 15 cm diameter and 31 cm height with a lateral connection to a tube of the same diameter. This allowed the growth of maize plants in the central compartment without direct exposure to aluminum, and the growth of AMF external mycelium towards the side compartment, where it would be exposed independently to Al. The compartments were separated by a nylon mesh with an opening of 24 μm, preventing roots but allowing the passage of AMF mycelium from the central to the lateral compartment.
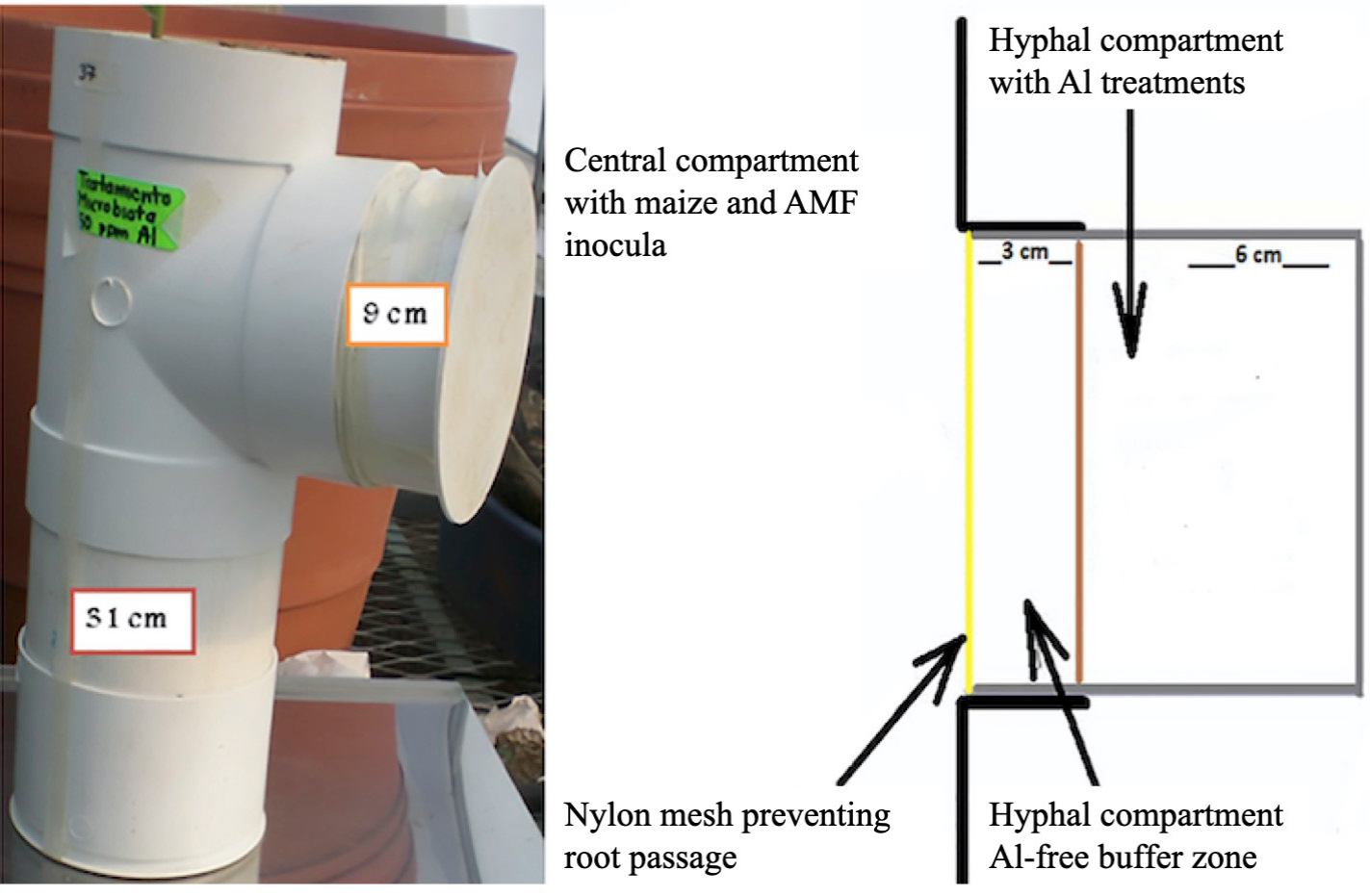
Figure 1 Growth system made of PVC tubes with a central and a side compartment where AMF external mycelium grew into the soil with the different Al treatments.
The soil with Al treatments was packed in the side compartment, leaving a 3-cm buffer zone space using soil without Al addition between the central and the side compartments that would prevent diffusion and mass flow of Al between compartments. Aluminum is an element with very low mobility in soil, which ensures that the Al placed in the side compartment remained there throughout the experiment. AMF inocula were placed in two layers in the central compartment alternating with layers of the soil:sand mixture without Al. The inoculum consisted of 150 g of soil and roots of the propagation pots and represented 5 % of the soil mass in the central compartment (3 kg). The control treatment received a mixture of the three inoculants autoclaved twice. Maize seeds NB9, a cultivar used in the región and verified to be widely colonized by AMF were disinfected for 10 min with a 10 % sodium hypochlorite solution. They were pregerminated for three days and planted placing three seeds in each system to ensure that at least one established. Then they were thinned to one plant per pot after emergence.
The pots were kept in a greenhouse and watered by weight at 70 % of their field capacity. Each time the plants were watered, they were rotated in the green-house tables to avoid systematic effects of microenvironmental conditions. Mean nighttime air temperature was 15 oC and mean daytime air temperature was 25-30 oC whereas light intensity and photoperiod (12.5 h) were natural. A NH4NO3 solution containing 50 mg N and a KH2PO4 solution with 10 mg P per kg soil were applied one month after starting the experiment to avoid nutrient deficiency and pot bounding. The plants remained in the greenhouse from April to July, to allow for the establishment of the mycorrhizal association in roots and for the development of external mycelium into the side compartment.
Harvest
After 92 days, the plants were harvested by cutting the maize shoots at soil surface and drying in the oven until constant weight. The side compartment and the nylon mesh were subsequently removed, and the soil was emptied into a tray. It was mixed to homogenize and extract a 50 g soil sample which was immediately placed in the freezer at -20 oC. The root was removed and washed until clean. It was weighed in fresh, then a fresh sub-sample of 2 g was taken for root colonization analysis, and the rest was placed in paper bags to dry in the oven to calculate and subtract water content.
Root colonization was measured by counting vesicles, hyphae, mycelium and arbuscules. The roots were clarified and stained with trypan blue according to Phillips and Hayman (1970). Root colonization percentage was determined under the microscope using the intersection method of Giovanetti and Mosse (1980). The AMF mycelium that developed in the lateral compartment was quantified with two methods. The first was through the technique of extraction, staining and quantification by microscopy (Jakobsen et al., 1992). To determine hyphal length, all intersections between the hyphae and the lines of a 1-mm grid area were counted in 25 fields of view. Although the soil was autoclaved, the control treatments presented also some mycelium, which can be dead mycelium of the native fungi that did not degrade completely after autoclaving, or live mycelium from saprotrophic fungi that may enter through irrigation water. As the microscopy method does not allow distinguishing dead and alive mycelium, the mean of the mycelium measured in control pots (which sums up the previous mycelium dead, but still present, and the possible growth of saprotrophic fungi) was subtracted from the measured values in the other treatments to correct for this residual mycelium and estimate the growth of AMF hyphae. The second method was the fatty- acid biomarker technique, which was performed with a four-step procedure according to Frostegard and Bååth (1996): soil extraction from frozen samples, lipid fractionation, alkaline methanolysis and gas chromatography analysis. Fatty acids were identified according to their retention time relative to a known standard. The fatty acid biomarker16:1ω5 was used to estimate the abundance of AMF (Olsson, 1999). This method, in contrast to the microscopy method, quantifies fatty acids present only in live cells, and is a measure of abundance in biomass, indicated by the phospholipid (PLFA) fraction, or in storage lipids, indicated by the neutral lipid (NLFA) fraction that accumulates in fungi but not in bacteria. In this study we used both the PLFA and NLFA fractions to estimate AMF biomass in soil, although the literature recommends the use of the NLFA fraction which excludes confounding bacterial lipids, because we expected a low amount of NLFA given the short time of the experiment. The NLFA fraction is expected to accumulate as AMF senesce and accumulate more storage lipids and this fraction represents indeed a cleaner biomarker than PLFA. We also used the NLFA/PLFA ratio of biomarker16:1ω5 to explore stress in the presence of Al in soil as, when fungi are under stress, they allocate more resources to storage (NLFA) than to biomass (PLFA) (Bååth, 2003). In addition, to determine if Al had affected other soil microbiota associated to AMF hyphosphere, PLFA biomarkers 18:2ω6,9 and 18:1ω9 were used to quantify the abundance of saprotrophic fungi and -iso, -anteiso, -OH, -cyclo, -methyl, and16:1ω7 fatty acid biomarkers derived from the same analysis were used for bacteria (Frostegard and Bååth, 1996; Ruess and Chamberlain, 2010).
Maize shoot samples were finely ground for phosphorus and nitrogen determination. The sample was digested with micro-Kjeldahl digestion (Bremner, 1996). The extract was read by colorimetry in a Bran-Luebbe autoanalyzer (Jackson, 1982). Three randomly selected maize shoot plants from each treatment were finely ground and analyzed for Al concentration after microwave digestion by Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
All data were processed using a two-way variance analysis, evaluating the effects of Al level and AMF inoculation treatment. Data were transformed as required, to meet the assumptions of the analysis. When the differences were significant, comparisons were made using a Tukey test. The analyses were conducted with JMP 8.0.
Results
AMF inoculation treatments had highly significant effects on most measured variables, but aluminum levels did not affect the development of AMF, hyphosphere biota, or maize plants and their nutrient concentrations (Table 1). Nor were significant interactions found between the two factors. Therefore, the values of the three levels of Al were pooled for all variables.
Table 1 Probability of significance values from the analysis of variance that assessed the effects of the different AMF inocula, aluminum levels and the interaction of the two factors, on AMF development variables and maize development and nutrition
| Variables | AMF inoculation | Aluminum level | I x A |
| AMF intra-radical colonization | <0.001 | 0.62 | 0.93 |
| AMF external mycelium length density | <0.001 | 0.71 | 0.46 |
| Soil total bacteria PLFA | <0.001 | 0.89 | 0.35 |
| Soil total saprotrophic fungi PLFA | <0.001 | 0.42 | 0.11 |
| Soil AMF 16:1ω5 PLFA | <0.001 | 0.35 | 0.4 |
| Soil AMF 16:1ω5 NLFA | <0.001 | 0.89 | 0.34 |
| NLFA 16:1ω5/PLFA 16:1ω5 ratio | <0.01 | 0.71 | 0.23 |
| Maize shoot dry weight | <0.001 | 0.3 | 0.056 |
| Maize root dry weight | <0.001 | 0.07 | 0.87 |
| Maize total dry weight | <0.001 | 0.2 | 0.15 |
| Shoot N concentration | <0.001 | 0.56 | 0.5 |
| Shoot P concentration | <0.001 | 0.18 | 0.59 |
| Shoot Al concentration | 0.11 | 0.77 | 0.52 |
The three inocula produced more than 20 % intraradical colonization. The inoculum with the lowest percentage of colonization was the native community of Al-contaminated soil, whereas A. delicata and G. margarita had similar values, of 30 to 60 % (Figure 2A). Controls did not show colonization. The inoculum of A. delicata produced the highest density of external mycelium, followed by G. margarita, and the native community inoculum had the lowest (Figure 2B).
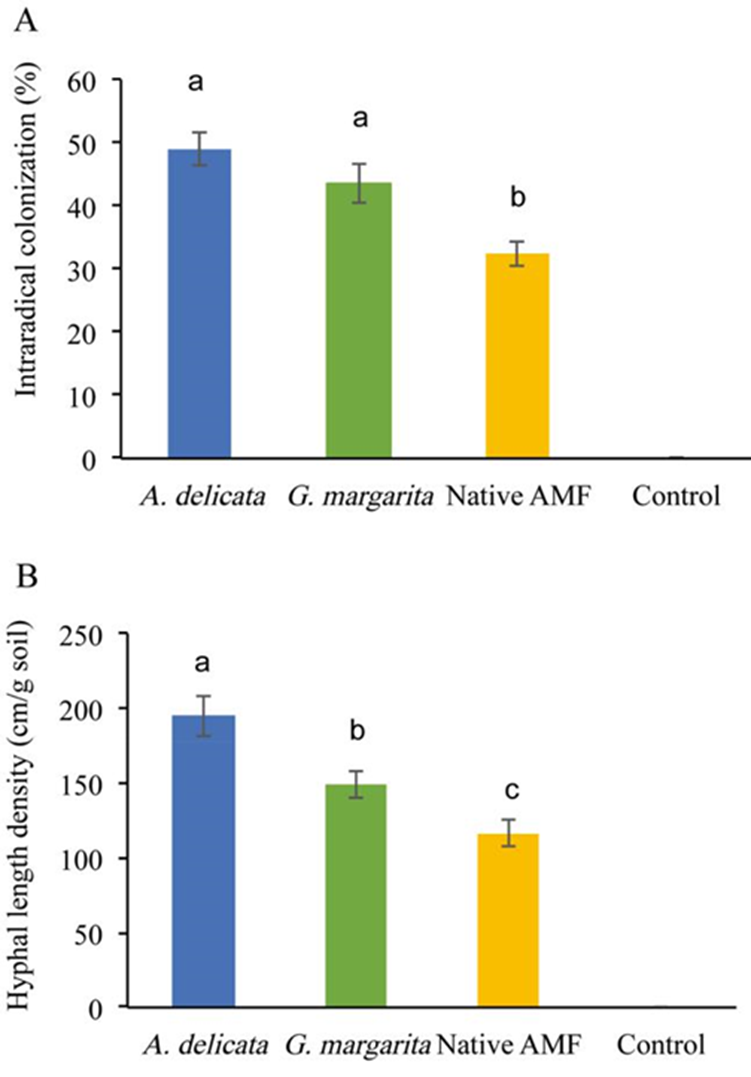
Figure 2 Percentages of intraradical mycorrrhizal colonization (A) and density of AMF mycelium in the hyphal compartment (B) measured at harvest. Different letters above the bars indicate a statistically significant difference between the treatment means. Means ± standard errors, n=15.
Pots with A. delicata inoculum also presented the greatest abundance of the biomarker of AMF and G. margarita and the native community had smaller amounts that statistically were not different from each other (Table 2). On the other hand, the NLFA 16:1ω5/PLFA 16:1ω5 ratio indicating physiological stress by favoring the accumulation of reserve lipids over biomass lipids, was highest in pots inoculated with G. margarita, followed by pots inoculated with A. delicata, whereas the native community showed the lowest ratio (Table 2). The control treatment showed a low value in both parameters likely due to the presence of other biota (mainly bacteria) in the soil. The inoculum of A. delicata was also the one with the greatest abundance of biomarkers for bacteria and saprotrophic fungi in the hyphosphere soil of the side compartment. The abundance of all microbial biomarkers was not affected by Al treatments but progressively decreased in inoculated treatments with G. margarita, to the native community, and the control, which presented the lowest values for all biomarkers (Table 2).
Table 2 Abundance (nanomoles g-1 dry soil) of microbial biomarkers for bacteria, saprotrophic fungi and AMF in the PLFA fraction, the AMF biomarker 16:1ω5 in the NLFA fraction and the ratio of the abundance of the AMF biomarker 16:1ω5 in the neutral fatty acid and phospholipid fatty acid (NLFA/PLFA) fractions, measured at harvest
| PLFA | NLFA | NLFA/PLFA | ||||||||
| Bacteria | Saprotrophic fungi | AMF | AMF | 16:1ω5 | ||||||
| A. delicata | 23.4 | (2.8)a | 4.9 | (1.6)a | 2.5 | (0.4)a | 25.4 | (2.9)a | 13.3 | (2.6)a |
| G. margarita | 17.3 | (2.2)a | 3.8 | (1.3)a | 2.4 | (1.2)a | 21.1 | (5.3)a | 20.5 | (5.9)a |
| Native AMF | 14.8 | (1.8)a | 3.2 | (1.1)a | 1.03 | (0.2)a | 6.0 | (2.5)b | 6.9 | (2.9)b |
| Control | 8.9 | (1.6)b | 1.9 | (0.7)b | 0.3 | (0.1)b | 1.7 | (0.8)b | 1.6 | (0.9)b |
Different letters within the same column indicate a statistically significant difference between the inoculation treatment means. Means ± standard errors, n=15.
Plants inoculated with the native community inoculum of the contaminated soil produced the highest shoot, root, and total biomass values and this was the only treatment that became significantly larger than the control (Figure 3). Plants inoculated with Acaulospora delicata did not differ from the control whereas plants inoculated with Gigaspora margarita were smaller than the control (Figure 3). There was a trend (data not shown, P=0.07) suggesting a reduction in root weight in treatments with 100 mg kg-1 of Al.
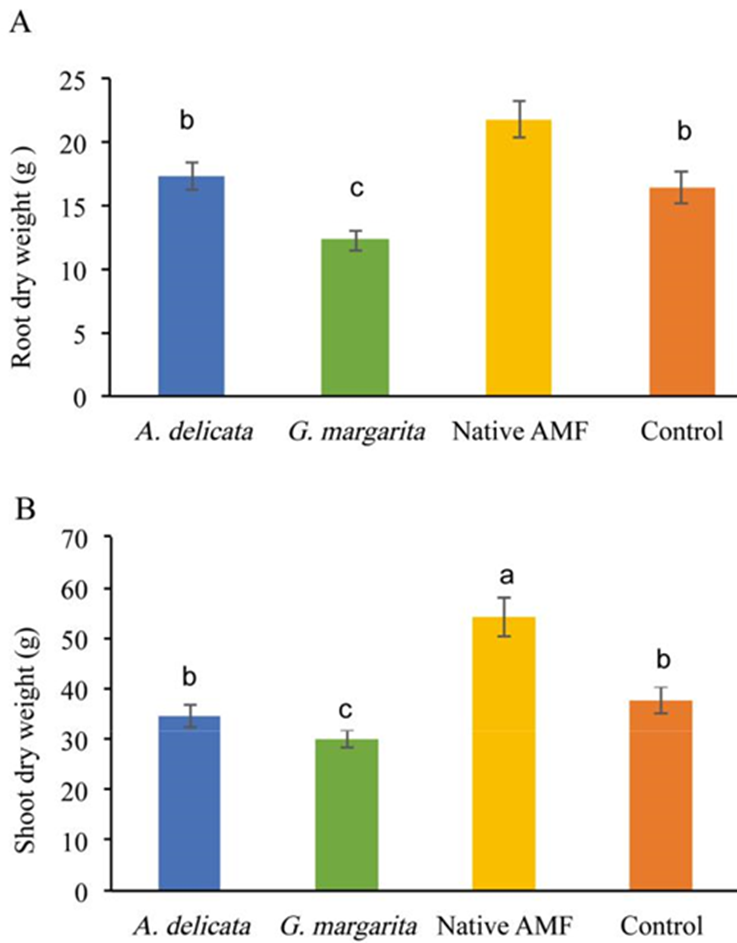
Figure 3 Root (A) and shoot (B) dry weight of plants with the four inoculation treatments. Different letters above the bars indicate a statistically significant difference between treatments Means ± standard errors, n=15.
Plants inoculated with A. delicata and G. margarita, and the controls, had similar shoot N concentration and plants with the native community had the lowest values (Figure 4A). The plants inoculated with A. delicata and G. margarita had twice as much P as the native population and the control (Figure 4B). Acaulospora delicata was the treatment that achieved the highest concentration of N and P and the only one that differed significantly from the control. The native community showed the lowest N and P concentration values, likely a dilution effect as this treatment achieved the highest biomass. There were no significant differences in the translocation of Al to maize shoots between inoculation treatments, or between Al levels added (data not shown). This may be due to the large variation observed in plants within the same treatment, with or without Al, which may be related to the small number of samples analyzed or the resolution of the method. Also, the Al values measured in the control plants that could not have obtained Al from the side compartment, varied also widely and fluctuated between 5 and 80 mg Al kg-1. As this large interval encompassed most of the values measured in the other treatments, there were no significant differences.
Discussion
Overall, the results indicated that concentrations of up to 100 mg Al kg-1 did not alter directly the growth of AMF in soil or in the roots. This is to our knowledge the first study that separates the effects of soil Al toxicity and acidity on AMF. This differentiation is important, because pH changes are known to alter the development of AMF (Van Aarle et al., 2002). Given that aluminum toxicity is closely associated with soil acidification, separating the two components would have been virtually impossible if we had used an already Al-contaminated soil and therefore with acidic pH. The soil used has high exchangeable Al and is thus prone to present Al toxicity problems if agronomic or other management reduced its pH (Casierra-Posadas and Aguilar-Avendaño, 2007), therefore it was important that we controlled its pH. The gradual addition of Al with minimal pH alteration of the soil allowed us to show that this element was not directly toxic at the levels studied and did not affect AMF or hyphospheric microbes’ abundance. There was though a trend suggesting that 100 mg Al kg-1 reduced root growth in maize. Al was physically separated from the maize plants by a 3-cm soil buffer zone and could have reached maize plants only by being transported to the central compartment by AMF mycelium. We measured Al concentrations only in maize shoots and found no effects of Al addition or AMF inoculation. We did not analyze roots because it is not possible to separate if Al actually entered maize plants or was retained in the intraradical mycelium. Kelly et al. (2005) found indeed up to two orders of magnitude more Al in roots than in shoots of Andropogon virginicus growing with additions of 400 µM Al to the soil, so it is likely that most Al is retained inside AMF hyphae and does not enter plant tissues. The trend observed in root reduction and the possibility of Al retention in AMF hyphae should thus be further analyzed in future studies.
These results agree with previous laboratory studies and reinforce the need for separating usually confounded effects of acidity, or effects on the host plant, from direct effects of metal toxicity on AMF. In experiments carried in vitro with transformed root cultures, we found a positive effect of Al on the development of the mycelium of Rhizophagus intraradices (previously Glomus intraradices), when pH was controlled and roots and mycelium were physically separated in different compartments (Méndez-Sántiz, 2009; Gavito et al., 2014). However, it was also observed that at a concentration of 20 μM Al, the roots showed significant reductions in growth. Similarly, in another pot study conducted with the same soil that was used in this study, but where both roots and AMF had contact with Al and pH was not controlled, the development of maize was more affected than that of AMF, at concentrations of up to 200 mg Al kg-1 (Saucedo-Correa, 2011). However, it was also observed that the intraradical colonization of a native community of the contaminated soil could develop better as Al increased, than other AMF isolates not previously exposed to Al. In our study all AMF tested were unaffected by Al and this can be due to the fact that we avoided pH changes and that the Al levels tested were low. Therefore, our prediction of higher tolerance of the native AMF community from an Al-contaminated soil to higher amounts of Al in soil was not supported.
It would be desirable to examine higher Al levels in subsequent studies, so that we can know at what levels AMF become affected. In our study, the soil used did not allow the Al concentration to be further increased without causing an abrupt reduction in pH, which was avoided as far as possible. The original pH of the soil (6) was reduced to 4-4.5 with Al additions beyond 100 mg Al kg-1 and that level of acidity would have significantly affected the development of AMF independently of the effects of Al.
Unfortunately, few studies have examined the response of fungi to metal exposure and even less to the increase in Al and there is little information to compare. As the focus is always placed on the plants, it is assumed that AMF are metal tolerant, and most measurements reported are plant variables. Altogether the evidence suggests that AMF can tolerate high levels of Al, even in the presence of soil pH changes, but plants are more sensitive to Al. It is known that the association of AMF and plant roots alters plant-soil interactions and improves plant growth under soil stress conditions (Kelly et al., 2005; Klugh and Cumming, 2007; Giovannetti and Gianinazzi-Pearson, 1994). Although it is known that AMF species have no specificity in the choice of their hosts, differences in the effects AMF species have on plant species growth indicate that plants vary in their response to different AMF species (Kobayashi et al., 2010). In this way, the performance of a host plant depends to a large extent on the type of AMF to which it is associated (Kobayashi et al., 2010; Chen et al., 2005). From our AMF inoculation treatments, Acaulospora delicata had the highest abundance in roots and soil and the most positive effect on maize nutrition and soil biota but it was the native AMF community from the Al-contaminated soil that best promoted maize growth. Gigaspora margarita, in turn, improved maize nutrition but actually depressed maize growth compared to the control, which suggests this species provides mineral nutrients but demands too much plant carbon.
On the other hand, we did not know how many AMF and other biota were present and colonizing when we inoculated the native community from an Al-contaminated soil, so the higher biomass could be due to all the microbiota introduced. Even though pot propagated AMF inoculum usually contains some biota from air-borne and irrigation water propagules, an agricultural soil has been influenced by many more plants, animals and microbes. The fact that the native community of Al-contaminated soil inoculum had the greatest effect on maize growth suggests that the accompanying microbiota promoted its development, as there was no evidence that this effect was due to a substantial nutritional improvement or to protection from Al toxicity by AMF. In addition to AMF, plant roots interact with diverse populations of soil microorganisms that have implications for plant growth and nutrition. In the legume-Rhizobia symbiosis, for example, there is also a wide variation in the tolerance of both plant and Rhizobia genotypes to Al, but in general plants alone are more sensitive to Al than the bacterial symbionts alone (Jaiswal et al., 2018). Soil nutrients released by other microorganisms associated with AMF may be transferred to the root surface through the rhizosphere, or through the mycorrhizosphere when in association with AMF (Richardson et al., 2009; Zhang et al., 2018). It was observed that the increase in Al did not affect the abundance of bacteria and saprotrophic fungi but the different inocula significantly changed the abundance of other soil microorganisms. Soil with single AMF species inoculants had higher abundance of AMF, saprotrophic fungi and bacteria when growing under identical conditions than the soil with native AMF community. This might be related to lower diversity and competition for resources in the single species inocula. The results highlight the importance of learning more about rhizosphere microorganisms and their interactions with other biota and abiotic factors. The presence of metals that are not essential for growth but can be taken up together with chemically similar elements that are essential nutrients is an important abiotic factor to keep exploring and understanding.











 nueva página del texto (beta)
nueva página del texto (beta)




