Introduction
The genus Pleurotus in Argentina is known mainly from morphological studies. Lechner et al. (2004) concluded that there are, so far, six species: P. albidus (Berk.) Pegler, P. cystidiosus O. K. Mill., P. ostreatus (Jacq.) P. Kumm., P. pulmonarius Fr. (Quél.), P. rickii Bres. and P. djamor (Rumph. ex Fr.) Boedijn (with three varieties: djamor, cyathiformis and roseus). The species have been found in the Pampas (growing on Populus and Salix trunks) and in tropical and subtropical regions (growing on Araucaria angustifolia and other tropical woods). Pleurotus ostreatus was only found in Patagonia growing on the native host A. araucana (Lechner et al., 2002). In Brazil Menolli et al. (2014) confirmed the presence of P. albidus, P. djamor, P. fuscosquamulosus D.A. Reid & Eicker, P. pulmonarius, and P. rickii with nrITS sequences study.
An accurate assessment of the genetic diversity of Argentinian Pleurotus wild strains is needed to establish their identities and allow accurate bases for their efficient use.
The white rot fungi Pleurotus spp. are characterized by their ability to degrade the lignin polymer of wood tissues. They produce a several oxidative enzymes including lignin peroxidase (LiP), manganese peroxidase (MnP), laccases and oxidases involved in the process (Mata et al., 2017). The laccases, a multicopper oxidases with low substrate specificity and strong oxidative ability (Wang et al., 2010) can be used for a variety of applications including paper pulp bleaching, deco lorization of textile dyes, transformation of lignin into its derivatives, and detoxification of harmful substances (Saparrat et al., 2008). The most efficient laccase-producers are white-rot fungi, such as Trametes versicolor (L.) Lloyd., Trametes hirsuta (Wulfen) Pilát and Pleurotus ostreatus (Šnajdr and Baldrian, 2007). For commercial applications, they usually require unique properties such as thermal stability and resistance to acid or alkaline conditions.
Enzyme activities show, in general, a progressive increase with temperatures till they reach the denaturation area, with the consequent (and sometimes irreversible) loss of activity. Coordination environment changes of the metal sites are also responsible for the loss of activity at high temperatures (Bonomo et al., 2001).
An important application of laccases in food industry is must beer and wine stabilization as an alternative to physical-chemical adsorbents (Osma et al., 2010). These complex mixtures of different chemical compounds have to be stabilized at low temperatures, increasing their shelf life, avoiding haze formation and preserving organoleptic madeirization-resistant features. These enzymes could increase productivity, efficiency and quality of food products without a costly investment and has the advantage of being a mild technology (Minussi et al., 2002).
Cold-adapted and thermostable enzymes are needed in industrial processes. Although low temperature conditions do not irreversible inactivate enzymes, their activities decrease to ranges in which they are not adequate for industrial processes (Cavicchioli et al., 2002). Patagonian microorganisms have adaptations to the stringent environmental conditions where they grow (Torres et al., 2016). These adaptations include the production of steroids, ergosterol (Dufourc, 2008), antioxidant compounds, carbohydrates, proteins and enzymes adapted to extreme cold conditions. Laccases of the edible mushrooms of the genus Pleurotus, growing naturally in Patagonia, could be used in different biotechnological applications such as food and beverage industries if they prove to provide cold-adapted enzymes.
The objectives of this work were (a) to elucidate the phylogenetic relationships of Pleurotus strains from Patagonia as a preliminary step in order to select those that are feasible to use in industrial processes that require cold temperatures and (b) to evaluate the laccase activity at low temperatures. We hypothesize that Patagonian strains retain a higher laccase activity at low temperatures than those from lower latitudes.
Materials and methods
Strains and culture media
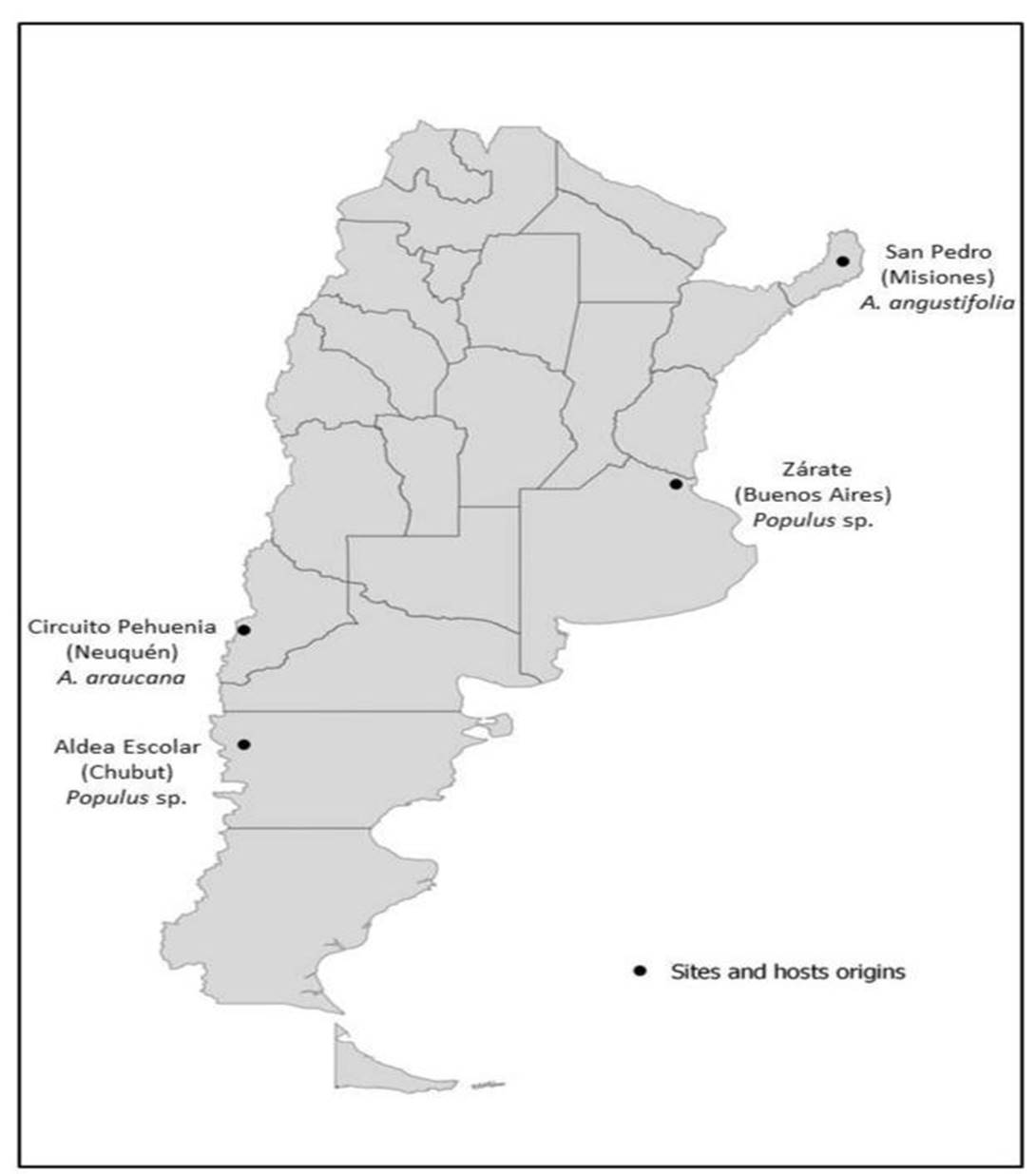
Eight strains of Pleurotus spp. were tested; they are kept at CIEFAPcc (Colección de Cultivos, Centro de Investigación y Extensión Forestal Andino Patagónico at the first author address) and at BAFC (Buenos Aires Facultad de Ciencias, Universidad de Buenos Aires Culture Collection). Locations of these strains are shown in Figure 1.
Pleurotus ostreatus, ARGENTINA, Neuquén, Villa Pehuenia, Paso del Arco a trail from Villa Pehuenia City to the international limit Paso del Arco with more than 50 kilometers from 1100 to 1400 m s.n.m., leg. M. Rugolo & M. Rajchenberg, May 3, 2013, CIEFAPcc 617, 621, 622, y 623; Circuito Pehuenia, Moquehue, a trail from Alumine City to Villa Pehuenia City with more than 100 kilometers (from 900 to 1300 msnm) leg. J. del Vas, March 3, 1994, CIEFAPcc 104. Growing on Araucaria araucana.
Pleurotus ostreatus, ARGENTINA, Chubut, Aldea Escolar, next to Arroyo Blanco, leg. M. Rugolo, June 26, 2013, CIEFAPcc 616. Growing on Populus sp.
Pleurotus pulmonarius, ARGENTINA, Buenos Aires, Zárate, leg. B. Lechner, May 10, 2011, BAFC 4281. Growing on Populus sp.
Pleurotus pulmonarius, ARGENTINA, Misiones, San Pedro, leg. E. Albertó, May 27, 2001, BAFC 263. Growing on Araucaria angustifolia.
The culture media used for mycelial growth was agar malt extract (Difco). Liquid cultures were prepared with 12.5 g of malt extract in 1 L distilled water in 250 mL flasks. The mycelium was incubated in static conditions at 23 °C in darkness (Rugolo, 2018). Supernatant was harvested after 30 days, when the colonization was completed.
Molecular determination
DNA extraction and PCR conditions. DNA was extracted from freshly collected mycelium from pure culture grown in liquid malt peptone broth with 10 % (v/v) of malt extract (Merck) and 0.1v % (w/v) Bacto peptone (Difco), in 15 mL tubes at 24 °C in the dark. DNA extractions were carried out with the UltraCleanTM Microbial DNA Isolation Kit (MO BIO Laboratories Inc., Solana Beach, California), following the manufacturers protocols. PCR for the full Internal Transcribed Spacer region (i.e., ITS1, ITS2 and the intervening 5.8S RNA gene; further referred as ITS) amplified with the primers ITS1 (TCCGTA GGTGAACCT) -ITS4 (TCCTCCGCTTATTGATATGC) (White et al., 1990). The PCR conditions were described by Imtiaj et al. (2011): 95 °C for 2 min, 30 cycles of 94 °C for 30 s, 51 °C for 30 s, 72 °C for 1 min, followed by 72 °C for 10 min. The amplified fragments were purified and sequenced at the DNA Synthesis and Sequencing Facility, Macrogen (Seoul, South Korea). Sequences generated in this study were submitted to GenBank (MK421536 to MK421541 and MK530228-MK530229).
Sequence and phylogenetic analyses.“Blastn” of the obtained sequences were performed against Genbank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Available ITS sequences of the P. ostreatus group obtained from previous studies (Vilgalys et al., 1996; Huerta et al., 2010; Menolli et al., 2014) were included. No outgroup was chosen, the tree being rooted to midpoint.
Nucleotide sequences for the ITS region were initially edited with BioEdit 7.0.9.0 (Hall, 1999), then aligned using L-INS-i strategy as implemented in MAFFT v 7.0 (Katoh and Standley, 2013) and manually adjusted using MEGA version 6 (Tamura et al., 2013). The final ITS dataset comprised 40 sequences and 569 characters including gaps. The bestfit models of evolution were determined using the AIC criterion (Akaike, 1974) implemented in jModelTest (Posada, 2008) and was GTR+G. Phylogenetic analysis of the data set was performed using Maximum Likelihood (ML) analysis was performed in PHYML executed on the south of France bioinformatics platform (http://www.atgc-montpellier.fr/phyml/) following Guindon et al. (2010) under the GTR nucleotide substitution model. Bootstrap values of the most likely tree were calculated with 1000 repetitions.
Laccase activity assays with Pleurotus strains
Laccase activity (E.C.: 1.10.3.2) was measured in supernatant using 2,6-dimetoxiphenol (DMP) 5 mM in 0.1 M sodium acetate buffer (pH 3.6) at 30 ºC, 10 ºC, and after heat treatment (the supernatant was exposed to 80 ºC, in thermal bath, for 20 min). Oxidation of DMP was determined by the increase in A469 (ε469=27 mM·cm−1) according Paszczynski and Crawford (1991). Retention of laccase activity at low temperatures was calculated as: [(activity measured at 10 °C / activity measured at 30 °C) * 100] (Yuan et al., 2016). Thermal stability was calculated as the percentage of remaining activity after thermal treatment measured at 30 °C. The measurements consisted in three replicates. Analysis of variance (ANOVA) were used with the measures of EU and the significant differences between treatments were compared by Tukey’s test at 5 % level of probability. The statistical treatments were tested by the software InfoStat software (Di Rienzo et al., 2011). Enzyme activity was expressed in International Enzymatic Units (EU) as the amount of enzyme required to release 1 μmol of product in 1 min.
Results and discussion
Molecular identification
ITS analyses including 40 taxa inferred from Maximum Likehood (ML) confirmed that all strains from Patagonia clustered within P. ostreatus species (94% Bootstrap value), while the strains isolated from center and NE Argentina belong to P. pulmonarius (89 %) (Figure 2). The latter has concordance with the tropical and subtropical distribution of that taxon as reported by Lechner et al. (2004) and Menolli et al. (2014).
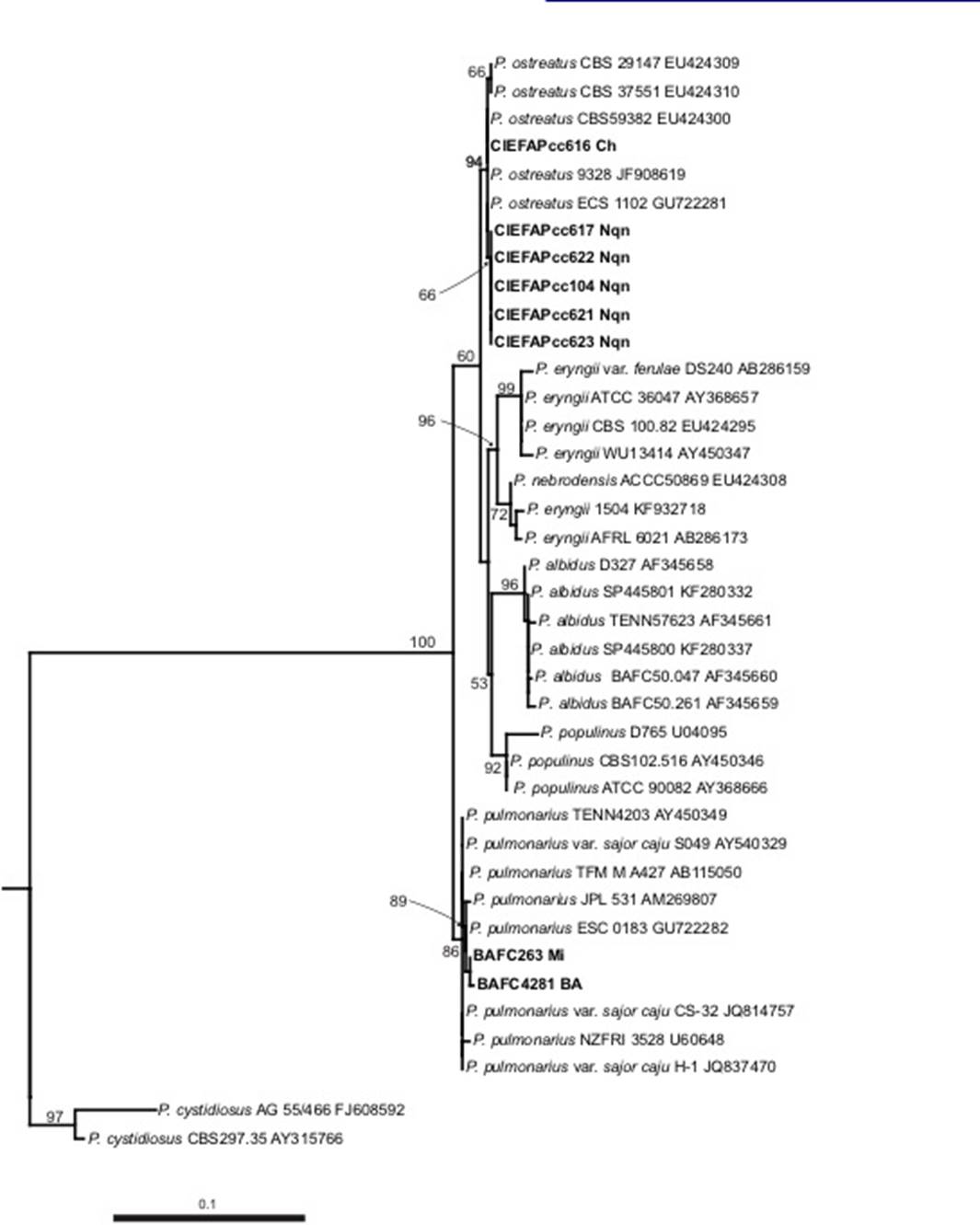
Figure 2. Phylogenetic tree of Pleurotus species based on rDNA-ITS sequences using Maximum Likehood analysis. Numbers on branches indicate the bootstrap values obtained from 1000 replications. Branches supported by bootstrap value greater than 50% are shown. CH, Chubut Province; Nqn, Neuquen Province; MI, Misiones Province; BA, Buenos Aires Province. Sequences in bold were generated in this study.
The description of Patagonian species based on macro-and microscopical features match with P. pulmonarius, but interespecific mating tests showed full compatibility with P. ostreatus (Lechner et al., 2002). Here we confirm the identity of the Patagonian strains as Pleurotus ostreatus.
According to Vilgalys (1997) the complex “Pleurotus ostreatus” groups P. ostreatus, P. pulmonarius, P. eryngii, P. abieticola, and P. populinus, which share a “pleurotoid” stature: more or less shelflike and radically eccentrically stipitate (Albertó et al., 2002). In our phylogenetic reconstruction (Figure 2) we agree with Albertó et al. (2002). Pleurotus phylogeny is currently based mainly upon ITS; this rDNA region solves the species relationships within the group but the branching pattern within P. ostreatus remains poorly determined. Liu et al. (2012) demonstrated that RPB2 gene to be superior to ITS and EF1α for distinguishing Chinese P. ostreatus. For a complete understanding of the genetic diversity of this species, including more Patagonian strains, future studies must include a multilocus approach using more variable gene regions and/or genotyping in order to assess population diversity.
It is a rarity that both species, P. ostreatus and P. pulmonarius, are found associated with conifers like Araucaria araucana and Araucaria angustifolia, respectively. The nature of the substrate points out towards the use of these strains for futures assays to evaluate growth and production of Pleurotus in resinous forest residues.
Laccase activity assays with Pleurotus strains
Strains from Neuquén presented the highest laccase activities with values close to 60 EU.L-1 for CIEFAPcc 104, 617, 621 and 622 (Figure 3). Strains of P. ostreatus 622 and 616, and strain of P. pulmonarius 263 and 4281 showed the highest retention of laccase activity at 10 ºC, with 70 %, 69 %, 58 % and 60 %, respectively (Figure 4), in comparison with their activity at 30 °C. Strain 623 presented the highest thermal stability after treatment at 80 °C (Figure 5).
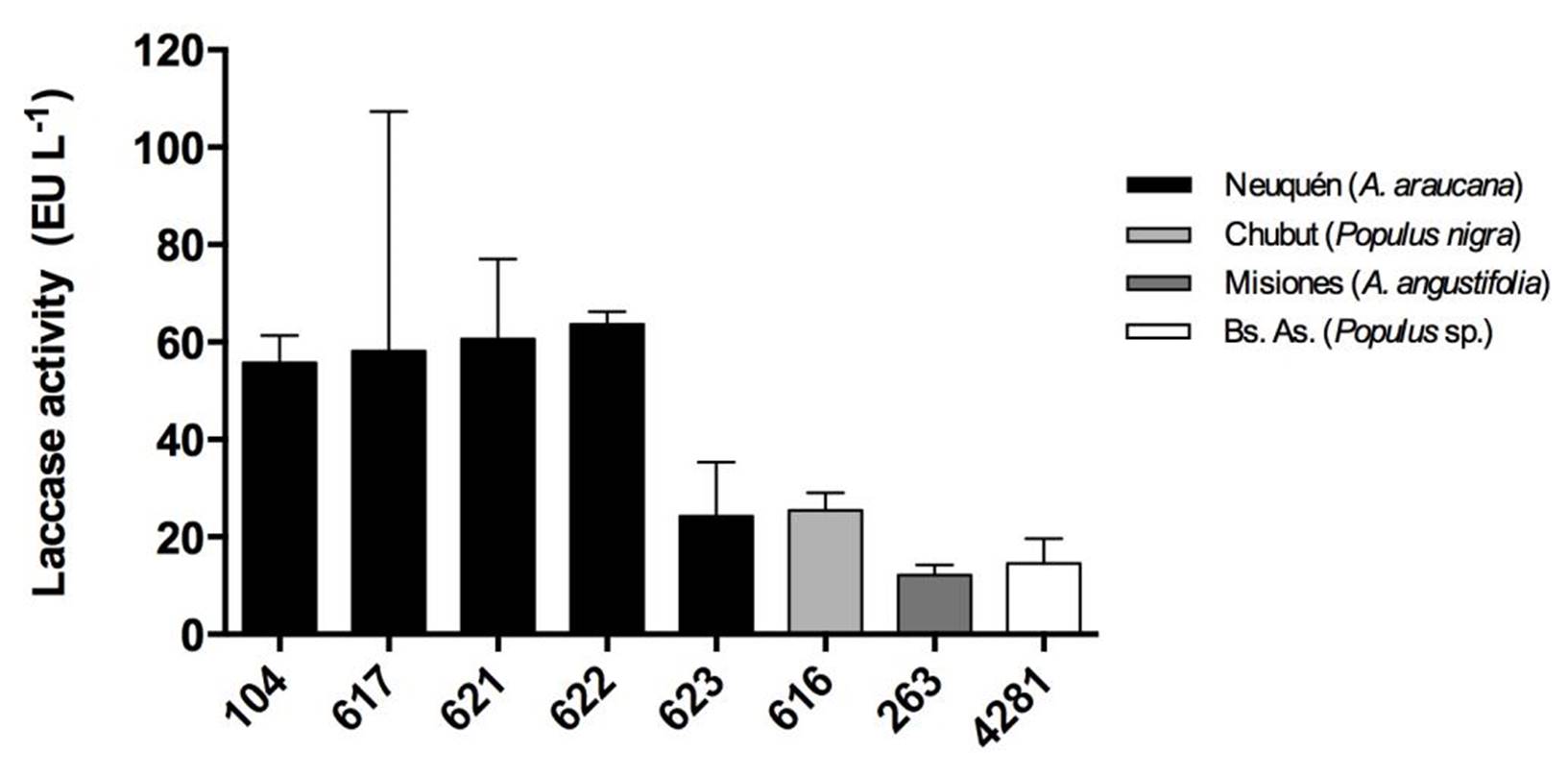
Figure 3. Laccase activity of the strains at 30 °C. Different numbers correspond to different Pleurotus ostreatus strains. Colors indicate the geographical origin of each strain. Means with the same letter are not significantly different (p>0.05).
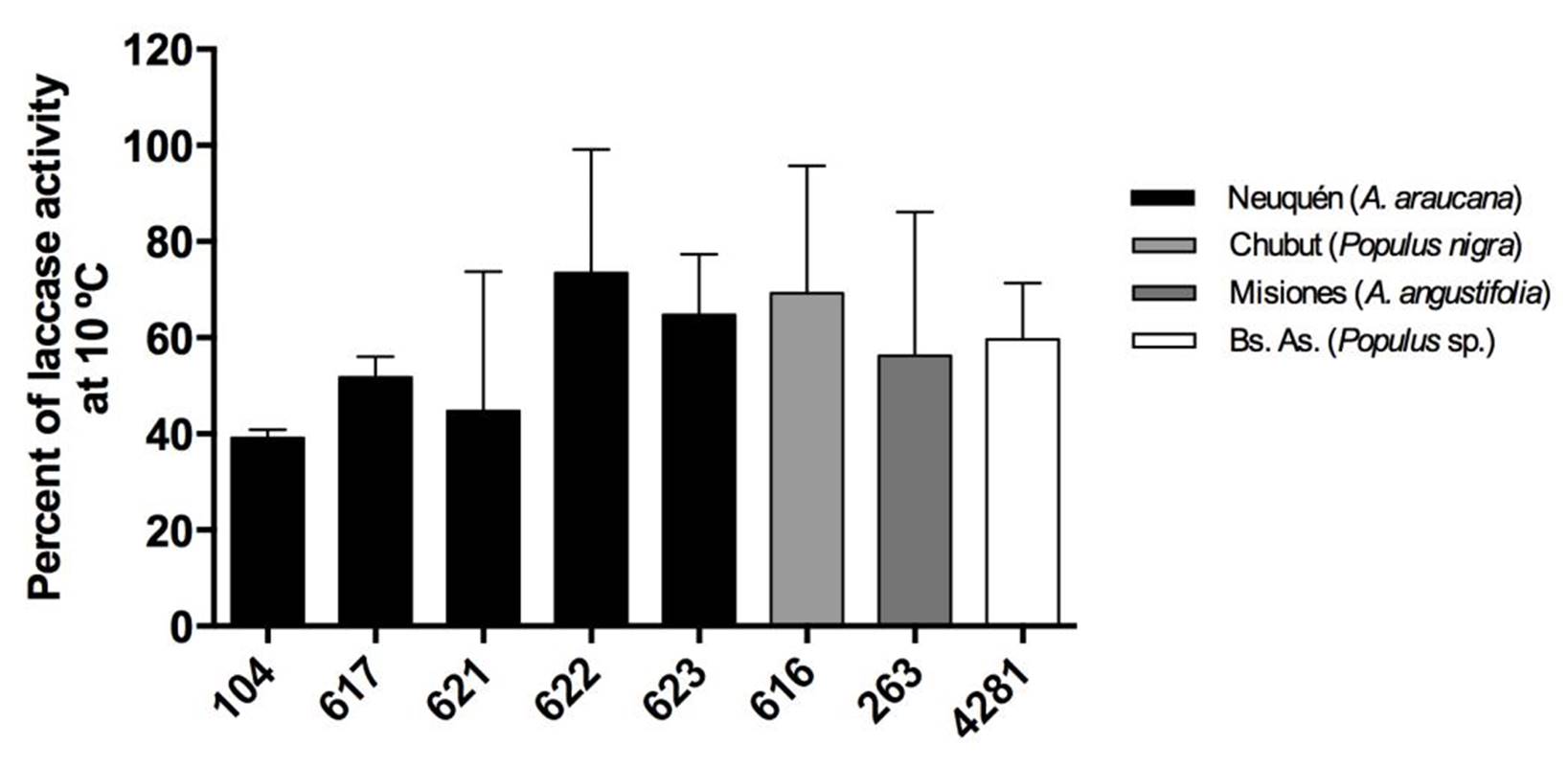
Figure 4. Percent retention of laccase activity at 10 °C. Different numbers correspond to different Pleurotus ostreatus strains. Colors indicate the geographical origin of each strain. Means with the same letter are not significantly different (p>0.05).
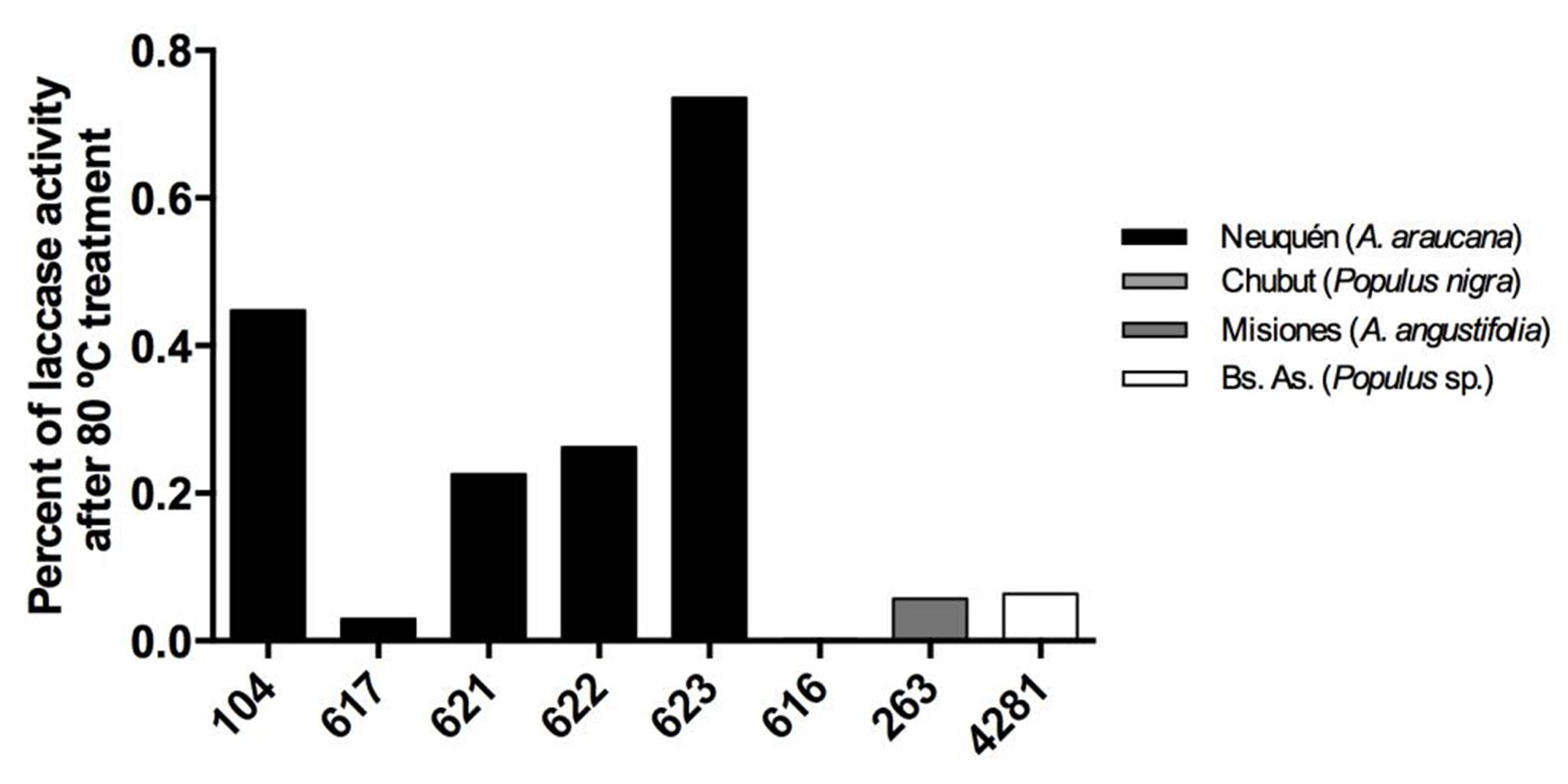
Figure 5. Thermostability of laccase activity at 30 °C, after heat treatment. Different numbers correspond to different Pleurotus ostreatus strains. Colors indicate the geographical origin of each strain.
Wang et al. (2010) showed better results with a Pycnoporus strain (from Jiangsu, China), with two purified stable laccases in a range of 0-50 ºC, and a remaining half-activity for 40 days at 30 ºC. When temperature exceeded 60 ºC Pycnoporus enzymes activities decreased rapidly, and they were inactivated after 1 incubation h at 80 ºC. Thermostability of the three laccase isoforms from Pleurotus nebrodensis was assayed by Yuan et al. (2016). They showed that Lac2 was the most thermostable and Lac1 demonstrated better thermostability compared with Lac3 in the temperature range from 30 °C to 70 °C. After exposure Lac2 to 50 °C for 120 min, 70 % of its original activity remained. A higher temperature of 70 °C, however, caused rapid inactivation (60 % and 80 % activity loss within 30 min and 60 min, respectively). Lac3 was completely inactivated at 70 °C after 40 min.
In comparison with our results, those purified laccases were more stable than those from P. ostreatus and P. pulmonarius strains from Argentina tested in this work. It should be noted that we worked with a total supernatant, in contrast with the purified laccases of Pycnoporus SYBC-L1 strain by Wang et al. (2010) and Pleurotus nebrodensis by Yuan et al. (2016). The total supernatant has a commercial benefit for industrial application, because it requires an enzymatic crude supernatant, for example, to precipitate phenolic compounds creating a completely clear juice, or without compromising the quality of beer or wine (Novozymes®).
Šnajdr and Baldrian (2007) showed the optimal laccase activity for a P. ostreatus strain from Czech Republic to be at 30 ºC with 24 EU.L-1, undergoing a great decline at low temperatures (0.02 EU.L-1 at 10 ºC), which also exhibited only 5-18 % of the activity at the optimum temperature at 10 °C. We have a notable difference with Patagonian P. ostreatus strains with more than 15 EU.L-1 at 10 ºC (more than 40 % of retention activity in all strains). Hua et al. (2018) showed that Pleurotus tuoliensis enzyme activity changed in response to cold stress. The transcriptional regulation of laccase and ligninolytic peroxidase genes plays an important role in the fruiting bodies of Pleurotus tuoliensis under low temperature induction (4 °C), in which the enzymatic activities and transcription levels decrease significan tly. At 13 ºC, the expression of laccase and peroxidase genes increased, and seems to play a dominant role during nutrition growth.
Smalas et al. (2000) proposed that high enzymatic efficiency at low temperatures is generally accompanied by reduced thermal stability because there is an increase in the molecular flexibility of the enzyme active site. However, in the system studied we observed that thermal stability did not decrease when laccase enzymatic activity at low temperature increased. Our results suggest that the supernatant produced by the studied strains can be used in processes at low temperatures but they also show a high thermal stability.
However, we rejected our hypothesis because there was no significant difference of activities at 10 °C between P. ostreatus strains from Patagonia and P. pulmonarius strains from north Argentina. We expected an association between strains and the environmental conditions. Nevertheless, laccase activity at 30 ºC and after thermal treatment was lower in Pleurotus pulmonarius (which grows in spring and summer of South Hemisphere) than in Pleurotus ostreatus (which fruits in autumn and winter season of South Hemisphere) that showed a similar percentage of activity retention at 10 ºC.
However, due to the low size number of strains, we cannot conclude easily on the effects of origin or species and the conclusion are bounded for the studied strains, but are not representative of the biodiversity of Patagonia.











 nueva página del texto (beta)
nueva página del texto (beta)