Introduction
Teratomas are cystic lesions that, depending on their degree of differentiation, help us determine the condition of malignancy for patients; being well-differentiated, they are given the name of “mature teratomas,” which are the result of a benign lesion; on the other hand, its poor differentiation attributes an undetermined malignant potential, these tumors are known as “immature teratomas.”
Approximately 75% of teratomas are well-differentiated and 12% are malignant and life-threatening; the remaining 13% corresponds to immature teratomas, whose damaging capacity is determined by the amount of immature tissue that has developed1.
The immature teratoma (IOT), whose name comes from the Greek term “monster,” is a rare, potentially cancerous tumor originating in germ cells, first described in 1960 by Thurlbeck and Scully. It occurs more often in reproductive age, highlighting mainly two incidence peaks: at 2 years of age and at the end of adolescence and early adulthood2; although they can appear at any age, they are unusual in pregnancy and potentially tend to be predominantly unilateral. Histologically, it is made up of three germinal layers (ectoderm, mesoderm, and endoderm), with the presence of immature epithelium and, in turn, they are classified according to their characteristics in tumor grades.
The presence of any poorly differentiated tissue at the germinal level is called a poorly differentiated teratoma, and the amount of immature nerve tissue determines the histologic grade, which is the most important predictor3. The prognosis is directly related to the histological grade of the primary tumor and its implants.
The treatment of malignant ovarian tumors is based on clinical stage and not tumor grade. Malignant teratomas frequently receive surgical treatment; in GRADE I, it consists of unilateral salpingo-oophorectomy with a large sample of peritoneal implants; in GRADES II and III, it is essential to complement the treatment with adjuvant chemotherapy.
The purpose of this report is to describe a case of peritoneal gliomatosis secondary to an immature teratoma, its management, analysis, and intervention.
Case report
27-year-old nulligesta patient, onset of menarche at 12 years, onset of sexual life at 15, with regular menstruations (5/30), a notable family history of cervical cancer on the part of the mother as well as both grandmothers, in their occupation, the patient reported a significant exposure to phosphates, denied chronic degenerative diseases. Among the gynecological antecedents, a history of non-oncological surgical intervention stands out in which a laparotomy and left salpingo-oophorectomy was performed; the pathological anatomy reports revealed a 40% mature left teratoma, 60% immature, Grade 3, without implants on the external surface of the capsule and a diameter of 2 cm; after the intervention, the patient was discharged from hospital 4 days later without any other adjuvant treatment or tracing.
After a year, she returned for disabling pelvic pain, apparently due to extreme physical activity. Physical examination, the patient was found to be in apparent good general condition, with a distended abdomen, painful on palpation, and a palpable tumor in the right hemiabdomen; the pelvis and female external genitalia with normal appearance and configuration, a permeable vagina without alterations and a cervix in good condition, evaluated through colposcopy.
Next, we will describe chronologically the interventions that our patient underwent, as well as a review of the results of the laboratory tests that were performed to establish her diagnosis; starting with an external consultation from which his first surgical intervention was derived; one month after this, the tumor markers were reported in their laboratory tests: carcinoembryonic antigen of 2.9 ng/mL (VR < 2.5), CA 19.9 of 1.0 IU/mL (VR < 37), CA 125 of 17.70 IU/mL (VR < 30.2), alpha-fetoprotein 4.48 IU/mL (VR < 8), DHL 349.6 Ul/mL (VR < 460), FA 39.0 Ul/ml (VR < 120), and beta-HCG fraction 0.30 Ul/mL (VR < 9.0).
Apparently, the parameters remained within the reference values, so the decision was made not to monitor it in the long-term (Table 1).
Table 1 Results of tumor markers throughout the follow-up of the patient in the pre- and post-operative together with the reference values
| Tumor markers | Pre-operative | Post-operative | Reference values |
|---|---|---|---|
| Carcinoembryonic antigen | 2.9 g/dL | 2.8 g/dL | 13.0-16.0 g/dL |
| Ca 125 | 17.70 Ul/mL | 7.21 Ul/mL | 35.5-44.9% |
| Ca 19.9 | 1.0 Ul/mL | 1.0 Ul/mL | 24-38% |
| Alpha-fetoprotein | 4.48 Ul/mL | 2.36 Ul/mL | 0.0-8.78 UI/mL |
| Lactate dehydrogenase | 349.6 Ul/mL | 170.0 Ul/mL | 125-220 UI/mL |
| Alkaline phosphatase | 39.0 Ul/mL | 81.0 Ul/mL | 40-150 UI/mL |
| Beta-hCG | 0.30 Ul/mL | 3.72 Ul/mL | 0-9.0 UI/mL |
Pre-operative values were captured on May 23, 2020; post-operative values were captured on October 24, 2020.
The following month, due to the persistence of her symptoms, the patient went to the clinic, where a real-time endovaginal ultrasound was performed with a 9 Mhz endocavitary transducer, observing the right ovary’s shape, size, and adequate situation, measurements 38 × 27 × 21 mm with a volume of 11.2 cc with the presence of multiple simple follicular-type cysts measuring between 5 and 8 mm, and the left one absent due to surgical history, observing in its topography an ovoid, isoechoic nodule that shows no sign of vascularity that it measures 47 × 30 × 13 mm, with a volume of 10.4 cc associated with free fluid in the fornix, measuring up to approximately 70 mL at its maximum diameter; suspicion data were the presence of significant magnitude of free fluid in the pelvic cavity.
The procedure performed subsequently corresponded to optimal secondary cytoreduction where a bilateral pelvic lymphadenectomy lumpectomy and partial omentectomy were performed; performing a pathological anatomy analysis of three flasks (Tumor on the right ovary surface, partial omentectomy, and right pelvic lymph) that reported a tumor on the surface of the right ovary, referred to as a mature teratoma, consisting of an irregular fragment of yellow red color, in which it measures 3.5 × 2.0 × 1.0 cm with a soft appearance on cut and a partial omentectomy, referred to as gliomatosis peritoneal, consisting of an irregular yellowish fragment that measures 19.0 × 14.0 × 1.0 cm, on cut, soft appearance; The definitive study obtained: peritoneal implants, consisting of multiple irregular fragments of purple tissue with white areas, of a soft consistency, which together measure 6.0 × 4.5 × 2.0 cm.
In the microscopic findings of the pathological anatomy, it was found that the tumor on the surface of the right ovary, microscopically composed of keratinized stratified squamous epithelium similar to that of the skin, in addition to mucosecretory ciliated cylindrical epithelium, likewise sebaceous glands, hair, tissue mature adipose, and few fragments of bone and mature cartilage, no immature components were reported in the examined material. In the histological sections of the partial omentectomy, fibroadipose tissue was observed with the presence of pilosebaceous glands, respiratory epithelium, enteric epithelium, cartilage and squamous, extensive mature glial tissue, and immature neuroectodermal tissue with rosette formation; likewise, in the histological sections of peritoneal implants, the same elements described in the omentectomy were evidenced and the histological sections corresponding to the peritoneal implants analyzed fibroadipose tissue with the presence of pilosebaceous glands, respiratory epithelium, enthetic epithelium, cartilage and squamous, glial tissue, adipose tissue, and immature neuroectodermal tissue (Fig. 1A-F).
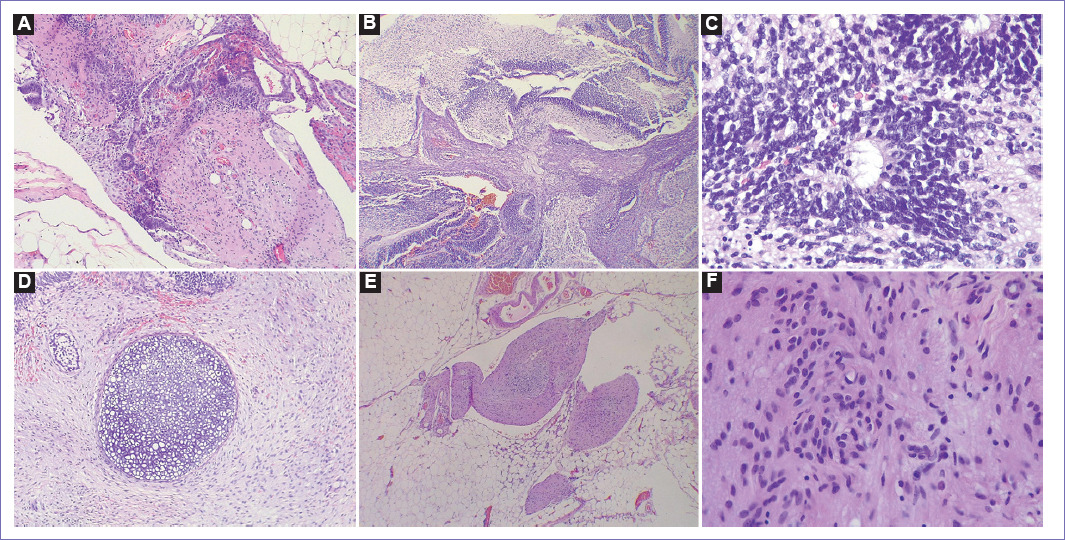
Figure 1 Photographs corresponding to histological sections of omentectomy and peritoneal implants. A: Adipose tissue with glial component and primitive neuroectodermal with solid pattern and rosette formation, ×5. B: Immature neuroepithelium component (hyperchromatic and hypercellular basophilic areas), alternating with hypocellular glial areas (eosinophilic areas), ×5. C: Immature neuroepithelium with rosette formation, ×40. D: Hyaline cartilage component surrounded by fibrous stroma, ×10. E: Mature glial component surrounded by mature adipose tissue, ×5. F: Mature glial component with fibrillar bottom, ×40.
Subsequently, it was decided to perform a brain magnetic resource imaging (MRI) to rule out a brain teratoma, and although it is a rare location, its deliberate search was carried out. This confirms: morphology of the skull and bone structures preserved, supratentorial and infratentorial ventricular system and the cisterns of the base with preserved caliber, with adequate differentiation between gray and white matter, amplitude of turns and normal fissures, basal ganglia, mesencephalon, medulla oblongata and the brain such as orbits, sella turcica, petromastoid regions, and craniocervical junction without evidence of alterations; in the 3D TOF images, vascular structures that make up the circle of Willis with preserved morphology are identified; In the post-contrast sequences, no abnormal enhancements are identified in the brain parenchyma. The paranasal sinuses are not evaluable for artifact. In conclusion, no alterations were found within the findings of this study.
With the findings obtained, the therapeutic decision was made to provide the patient with four cycles of chemotherapy with bleomycin, etoposide phosphate, and cisplatin; as a control, tumor markers were performed and these remained at normal values: carcinoembryonic antigen of 2.8 ng/ml (VR < 2.5), CA 19.9 of 1.0 IU/mL (VR < 37), CA 125 of 7.21 IU/mL (VR < 30.2), alpha-fetoprotein 2.36 IU/mL (VR < 8), DHL 170.0 Ul/mL (VR < 460), FA 81.0 Ul/mL (VR < 120), and HCG beta fraction 3.72 Ul/mL (VR < 9.0) (Table 1).
The patient was treated with BEP adjuvant therapy: bleomycin etoposide and paclitaxel, and adjuvant treatment was based on clinical stage and symptomatology, not patient-specific tumor grade; initially, a high genetic load of cancer was attributed to him, which, together with the risk factors associated with phosphate exposure, is considered the key point for the development of his disease.
The study of response to treatment was with PET-CT where data on hypermetabolic tumor disease in the neck (SUVmax: 2.6), mediastinum (SUVmax: 4), internal mammary chain (SUVmax: 2.2), and liver (SUVmax: 3.3) were determined, peritoneal implants (highlighting the cecal appendix) (SUVmax: 14), data that may suggest secondary deposits, right rectus abdominis muscle, and solid implant in front of the bladder (SUVmax: 18.8).As well as an image suggestive of ovarian teratoma behind the uterus (SUVmax: 3.3). The rest of the biodistribution of the radiotracer is considered physiological (brain, Waldeyer’s ring, myocardium, liver, spleen, intestinal system, and urinary elimination route). In conclusion the highly suspicious tumor was located in peritoneal, bladder and mediastine.
Discussion
Germ cell tumors mainly affect prepubertal and pubertal youth; immature teratomas are frequently associated with ectodermal sinus tumors and dysgerminomas4. Within genetic studies, the presence of the Y chromosome has been found in the karyotype of some pediatric patients with gonadal dysgenesis, which is a risk factor for developing a dysgerminoma5. Although these tumors are endocrine dependent, elevated serum levels of lactate dehydrogenase, alkaline phosphatase, or both are found in 95% of dysgerminoma cases.
Peritoneal gliomatosis is a rare form of extension or metastasis of immature teratomas. This causes the formation of a graft of glial tissue in the peritoneum or another organ and has been described with a higher incidence in the second decade of life.
Due to the infrequency of peritoneal gliomatosis, no single accepted guide has been issued on treatment, time, and follow-up of patients who suffer from it, so it is necessary to make an accurate diagnosis with the help of different biomarkers, such as GFAP, protein S-100, and neurospecificity enolase to determine the presence of mature and well-differentiated glial tissue6.
Although gliomatosis peritoneal by itself has a low malignant potential, surveillance, and strict follow-up by means of evolutionary controls are still necessary; alpha-fetoprotein is usually a serum marker used for the diagnosis and follow-up of germ cell tumors; however, it has been shown to have low specificity and sensitivity for the follow-up of patients with ovarian teratomas (since its concentrations may be within the normal limits), although it is especially useful for ruling out metastases from immature ovarian teratomas7. The study of the response to adjuvant treatment must be with PET and subsequent surveillance with the same PET to determine recurrence.
The treatment consists mainly of exploratory laparotomy tumor resection, with systematic review of the abdominal cavity with peritoneal fluid sampling for cytological study and biopsies of peritoneal implants. In cases associated with mature ovarian teratoma, surgical removal of the primary tumor may be sufficient8. On the other hand, in cases associated with immature teratomas, it is necessary to carry out complete staging and take samples from as many implants as possible, since clinical management and prognosis depend or not on immature glial elements in the implants; if these elements exist, the patient should be treated and monitored as in cases of metastatic ovarian carcinoma. Adjuvant treatment options include the combination of bleomycin, etoposide, and cisplatin or vincristine, adriamycin, cyclophosphamide depending on the accessibility of medications and the patient’s clinical condition. The 5-year prognosis is related to between 30% and 82% depending on the surgical staging, in addition to early detection and timely treatment, as well as good management by the treating physicians and consultation with a specialist to provide comprehensive patient care.
Likewise, one of the main challenges during the care of this patient was her circumstances, such as her age, marital status, and nulliparity; remembering that for many women motherhood is a transcendent function in their lives that have an impact on the social, affective, and psychological spheres, for which it is necessary to take into account their preferences and based on them, provide them with adequate treatment that does not compromise your family planning and freedom of choice through conservative treatment, leaving in the future the possibility of a complete treatment once the desired maternity has been satisfied; seeking to preserve fertility, a resection of the main tumor, and a study of lymphatic involvement and support with simultaneous therapies are performed; The treatment of choice is based on providing adjuvant chemotherapy, so it is important to consider it within the therapeutic possibilities and adaptations to the patient.
Establishing the differentiation is vital to proceed with the appropriate treatment; additionally, immature teratomas have the capacity for recurrence and distant dissemination, where one of the most frequent locations are peritoneal implants, as occurs in the case of gliomatosis peritoneal, which has a predominance of glial tissue and is characterized by having an unfavorable prognosis, as previously stated in the review of this clinical case.











 nueva página del texto (beta)
nueva página del texto (beta)


