Introduction
Ionizing radiation (IR) is the energy transported through rays emitted by radioactive material, X-ray emitters, or tomographs. Radiation from alpha and beta particles, X-rays, and gamma rays provokes a biological response due to the ionization of cellular molecules1. Depending on the dose and time of radiation exposure, IR increases the risk of developing cancer, as it interacts with DNA, generating mutations and triggering pro-carcinogenic metabolic pathways1,2.
Modern diagnostic images have made it possible to diagnose pathologies that cannot be seen with the naked eye or by laboratory investigations. One of the most used techniques is computed tomography (CT), which usually has high sensitivity and specificity3,4. The high doses of IR related to CT, however, have been associated with an increased risk of developing malignant neoplastic processes5 such as leukemia and brain tumors6. The available medical evidence on the risk attributable to the use of CT and the risk of cancer is, however, controversial. Some studies affirm that this risk is low or null with low-dose radiation in patients required to undergo this diagnostic technique regularly3-7. The increase in the use of this diagnostic technique in recent decades calls for the need to explore potential side effects, and if possible, minimize the frequency and dose of radiation exposure.
Two hypotheses have been described regarding the pathophysiology of radiation-induced cancer: a pre-existence of subclinical tumors undetectable to CT and individual susceptibility to cancer. This generates a greater risk of developing cancer, regardless of the dose or frequency of exposure to IR6. This review aimed to map the available medical evidence on the association between IR exposure when undergoing CT and the risk of developing a malignant and/or benign neoplastic process.
Methodology
A scoping review was conducted based on the steps proposed by Arkesy and OMalley8 and reviewed by Levac9: (a) define the research question; (b) search for the relevant medical literature; (c) selection of relevant studies; (d) data extraction; and (e) summarize and export the results. The review sought to answer the question: what is the nature and extent of the medical literature on the relationship between IR emitted by a CT scan and the development of neoplastic events?
Eligibility criteria
A inclusion criteria were established: (a) the language of the publication was Spanish or English; (b) articles published in any year; (c) observational epidemiological studies (case control, cross-sectional, and cohort studies); and (d) documents that explore the association between radiation by tomography and the appearance of neoplastic events. We excluded theoretical publications (narrative reviews, letters to the editor, editorials, and opinions) and articles without access to the abstract or the full text. The last update of the search strategy was August 11, 2021.
Information sources and search strategy
PubMed and Scopus databases were used for this review. For the search strategy, Boolean operators and keywords were used according to each data system. The search algorithm is available in Supplementary File 1. In addition, the references cited in the reviewed documents were included if they met the inclusion criteria and if they had not been previously considered.
Study selection and data extraction
The titles and abstracts of the candidate publications were independently reviewed based on the eligibility criteria. Discrepancies on the inclusion were solved by discussion and consensus, involving a third party if needed. Subsequently, duplicates were eliminated, the full-text version of the documents was obtained, and the final selection was made based on the inclusion criteria.
Synthesis and presentation of results
The results are presented following the categories proposed by Graniewicz et al.10: (a) a summary of the characteristics and distribution of the included publications (authors, type of document, characteristics of the population, objective, type of cancer, journal, country of the authors, and main findings/contribution) and (b) a narrative synthesis of the most relevant manuscripts. This article used the PRISMA extension to report exploratory systematic reviews (PRISMA-ScR)11. The completed checklist is available in Supplementary File 2.
Results
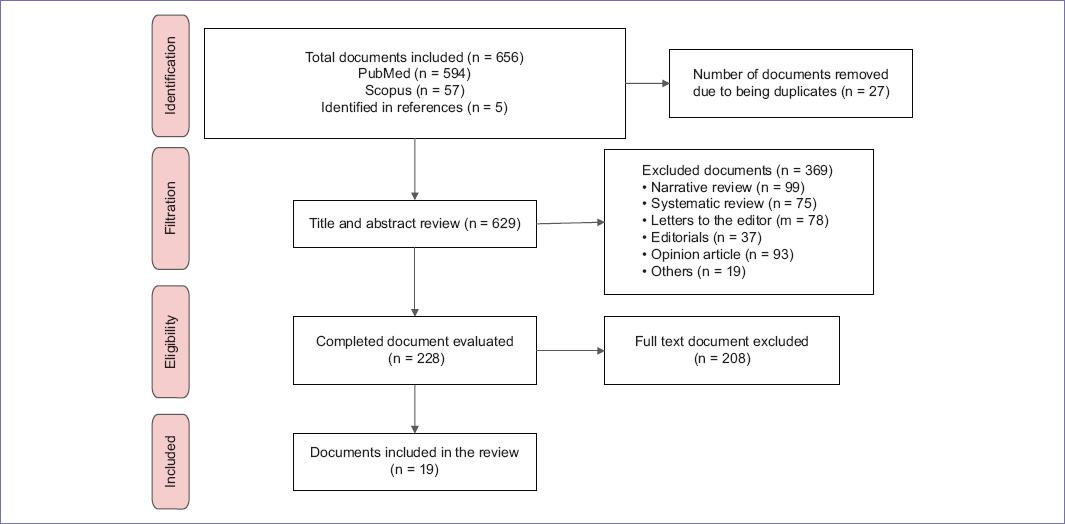
Nineteen publications (17 retrospective cohort studies and two cross-sectional studies) were included in figure 1 and Supplementary File 3. The author countries were the United States (n = 11), South Korea (n = 3), England (n = 2), Greece (n = 1), Switzerland (n = 1), and Australia (n = 1). The neoplastic events described in the publications included brain, lung, gastrointestinal, thyroid, mammary gland, and pharynx cancers, as well as leukemia and melanoma. The general characteristics of the documents are found in table 1.
Tabla 1 Characteristics of articles exploring
| Authors | Document Type | Population characteristics | Objective | Type of cancer | Journal | Country of the authors | Main finding/contribution |
|---|---|---|---|---|---|---|---|
| Lee et al. | Prospective cohort | 825,820 men and woman; average age of 28 years | To assess the risk of developing hematological malignancies in a patient undergoing appendectomy for acute appendicitis | Leukemia | JAMA Surgery | South Korea | The study population exposed to CT had a high risk only for leukemia (RR: 1.40; IC del 98.75%: 1.04-1.87; p = 0.05) |
| Mathews et al. | Retrospective cohort | 10.9 million men and women aged 0-19 years | Assess the risk of CA in children and adolescents after exposure to low doses of IR when exposed to CT | Melanoma Leukemia Myelodysplasia | British Medical Journal | Australia | The incidence of CA was 24% higher for those exposed to radiation by CT than for those not exposed.(RR: 1.24; IC del 95%: 1.20-1.29; p < 0.01) |
| Pearce et al. | Retrospective cohort | 355,191 men and women under 22 years of age | To assess the risk of leukemia and brain tumors after CT scanning in a cohort of children and young adults | Leukemia Cerebral CA | The Lancet | England | One case of leukemia is estimated (RR: 3.18; IC del 95%: 1.46-6.94) and a case of cerebral CA (RR: 2.82; IC del 95%: 1. 336.03) per 10,000 patients younger than 10 years exposed to CT |
| Koral et al. | Retrospective cohort | 182 men and women under 20 years of age | Estimating the LAR of developing CA due to exposure to CT versus MRI for the evaluation of ventricular size in children with shunt hydrocephalus | Cerebral CA | American Journal Neuroradiology | United States | CT for monitoring the ventricles with the frequency of two images per year between 2 and 20 years of age would produce one case of CA for life for every 97 patients exposed to IR |
| Kim et al. | Retrospective cohort | 365 men and women aged 0-18 years | Estimate the LAR of developing CA due to exposure to CT versus MRI for evaluation of ventriculoperitoneal shunt function | Cerebral CA | Pediatric Emergency Care | United States | MRI is an effective alternative to CT by reducing the risk of IR and developing CA induced by its exposure |
| Brenner et al. | Retrospective cohort | 600,000 CT examinations of the abdomen and head in girls and boys under 15 years of age | Evaluate the risk of mortality due to developing CA due to IR exposure by performing a CT scan | Leukemia GI, lung and breast CA | American Journal of Roentgenology | United States | The estimated risk of mortality in a 1-year-old child is 0.18% for GI CA and 0.07% for cerebral CA |
| Mazonakis et al. | Cross-section | Does not apply | Estimate the associated risk of developing thyroid CA in a pediatric population undergoing head-and-neck examinations with CT | Thyroid CA | Springer | Greece | A risk of developing thyroid CA of 4-65 per million and neck CA of 390 per million patients was evidenced |
| Smith- Bindman et al. | Cross-section | 1,119 men and women over 18 years of age | Estimate the IR dose of CT scans and quantify the potential risk of CA associated with these examinations | Does not specify | American Medical Association | United States | In the 20-year-old population, the risk of CA is double that of 60-year-old patients |
| Nordenskjold et al. | Retrospective cohort | 26,354 men and women | To assess the association between exposure to head CT and the risk of developing meningioma | Meningioma | Radiological Society of North America | United States | No increase in meningioma cases was found among subjects exposed to CT compared to unexposed (HR: 1.49; IC del 95%: 0.97, 2.30; p = 0.07). |
| Johnsons et al. | Retrospective cohort | 337 men and women≤6 years of age | Estimate the LAR of developing CA in a pediatric population with heart disease | Breast and thyroid CA | Circulation | United States | The associated attributable risk of CA is 0.07% (RI: 0.001 a 6.5%) |
| Wu et al. | Retrospective cohort | 505 men and women aged 21-80 years | Estimate the LAR of developing CA in the population exposed to dental CT | Leukemia, thyroid and lung CA | Journal of Dental Research | United States | The LAR for female thyroid CA peaked before age 45; presenting a risk up to 8 times higher than a 30-year-old woman compared to a 50-year-old |
| Huda et al. | Retrospective cohort | 100 men and women; average age 59±11 years | Estimate the risk of developing CA in the population exposed to cardiac CT angiography | Does not specify | American Journal of Roentgenology | United States | The risk of developing CA was 0.13% |
| Hong et al. | Retrospective cohort | 12,068,821 men and women between the ages of 0 and 19 | Estimate the risk of developing CA in the pediatric population exposed to low-dose IR by CT | Leukemia, myelodysplasia, thyroid and breast CA | JAMA Oncology | United States | A higher incidence of CA was evidenced in exposed patients compared to those not exposed. (IRR, 1.54; IC del 95%:1.45-1.63; p < 0.001) |
| Lim et al. | Retrospective cohort | 1.4 million men and women | Estimate the incidence and mortality from CA attributed to IR exposure given by diagnostic methods such as CT | Bladder, colon, liver, lung, stomach, thyroid, breast CA and leukemia | The Korean Academy of Medical Sciences. | South Korea | The fraction attributable to the population of the incidence and mortality due to CA was 1.2% and 1.7% in women, in men it was 0.6% for incidence and mortality |
| Jha et al. | Retrospective cohort | Men and women ages 5-40 | Estimate the LAR of developing CA attributable to IR of CT for orthodontic purposes in children and adults | Oral cavity, pharynx, esophagus, lung, CNS, thyroid, and BM CA | The Korean Journal of Orthodontics | South Korea | The risk of CA for children was 14.28% and 0.91% for adults; this indicated that the risk for children was 16 times greater than the risk for adults |
| Miglioretti et al. | Retrospective cohort | 4,857,736 children under 15 years of age | To determine the rate of use of CT scans in the pediatric population and the impact of this on the development of CA | Leukemia, Solid CA | National Institutes of Health | United States | Approximately 4 million CT scans are performed in the pediatric population, which will cause an estimated 4870 CA in the future |
| Wylie et al. | Retrospective cohort | 3,863 men and women over 18 years of age | Estimating the LAR of developing malignancy in young adult patients exposed to a hip/pelvic CT | Leukemia, Solid C | The Journal of Arthroscopic and Related Surgery | United States | Hip/pelvic CT scans have a risk of 0.105% and 0.177% for a 20-year-old man and woman, respectively |
| Huang et al. | Retrospective cohort | 24,418 men and women under 18 years of age | To assess the risk of developing benign and malignant neoplasia of the brain in pediatric population exposed to head CT | Malignant/benign brain tumor | British Journal of Cancer | England | The risk of benign brain tumor was significantly higher in the exposed cohort than in the unexposed cohort (HR = 2.97, IC del 95% = 1.49-5.93) |
| Kim et al. | Retrospective cohort | 11,072 men and women over 20 years of age | Estimate the risk of recurrence of GI CA, mainly gastric in early stage, in population exposed to recurrent abdominopelvic CT | Solid C | Cancers | Switzerland | A relationship was found between the frequency of performing a CT scan and the subsequent incidence of GI CA recurrences |
CT: computed tomography; CA: cancer; IR: ionizing radiation; MRI: magnetic resonance imaging; GI: gastrointestinal; CNS: central nervous system; BM: bone marrow; LAR: lifetime attributable risk; IRR: incidence rate ratios.
Retrospective cohort studies
Matthews et al.12 estimated the increased risk of cancer in the pediatric population aged 0-19 years exposed to CT IR. The cohort included 10,939,680 patients, of which 680,211 (6.2%) belonged to the exposed group. The mean duration of follow-up was 9.5 years for the exposed group and 17.3 years for the unexposed group. Adjusting for age, gender, and year of birth, the overall incidence of cancer was 24% higher in the exposed group compared with unexposed individuals (Incidence rate ratios: 1.24; 95% CI: 1.20-1.29). A statistically significant dose-response relationship was also described. Moreover, greater susceptibility to developing cancer was reported when exposed to IR at earlier ages (p < 0.01). The incidence significantly increased for neoplasms of the gastrointestinal tract, soft tissue, female genital organs, thyroid, as well as for melanoma, Hodgkin lymphoma, leukemias, and myelodysplasias.
Pearce et al.13 evaluated the impact of exposure to IR (one or more CT scans) on the risk of developing leukemia in 178,604 patients aged 0-15 years. The study also explored the risk of developing brain tumors in 176,587 patients aged 0-15 years. A total of 283,919 CT scans were performed, of which 64% were of the head, 9% of the abdomen and/or pelvis, and 7% of the chest. A total of 74 patients were diagnosed with leukemia and 135 with brain tumors. There was a positive association between the radiation dose of CT scans and the risk of developing leukemia (Incidence rate ratios per 50 mGy: 0.036; 95% CI: 0.005-0.120) and brain tumors (Incidence rate ratios per 60 mGy: 0.023; 95% CI: 0.010-0.049). Regarding this data, although the risk of developing leukemia and brain cancer was tripled depending on IR dose, the authors commented that the clinical benefits and the low radiation dose should be considered as a measure to mitigate the risk of developing cancer. Miglioretti et al.13 determined the lifetime risk of developing cancer of solid organs due to IR exposure by CT among the pediatric population, including mostly women. The development of one case of cancer was reported for every 300-390 CT of the abdomen/pelvis, 330-480 CT of the chest, and 270-800 CT of the spine.
Kim et al.14 evaluated 11,072 adult patients who underwent endoscopic or surgical resection for early-stage gastric cancer. The patients were exposed to 32,653 abdominopelvic CT scans for 5 years. Three hundred and twenty-two patients developed an additional primary cancer. Patients exposed to more than 8 CTs (Hazard ratio: 2.73; 95% CI: 1.66-4.50), older than 50 years (Hazard ratio: 2.64; 95% CI: 1.87-3.73), male (Hazard ratio: 1.61; 95% CI: 1.17-2.21), and ex-smokers or current smokers (Hazard ratio: 1.58; 95% CI: 1.23-2.03) were particularly at risk. Furthermore, additional CT scans were associated with the development of liver, pancreas, kidney, and bladder cancer (Hazard ratio: 1.14, 95% CI: 1.07-1.22). The authors concluded that the excessive use of CT in patients with gastric cancer increases the risk of developing a second primary intra-abdominal cancer.
Huang et al.15 investigated the association between exposure to one or more CT scans of the head and the risk of developing benign or malignant brain neoplasms in a cohort of 24,418 exposed subjects versus 97,668 subjects not exposed to IR. During the study period, 93.4% of the participants were exposed to one CT scan, 5.42% to two, and 1.20% to more than two images. The incidence rate of malignant and benign tumors was 36.72/100,000 person-years in the exposed cohort versus 28.48/100,000 person-years in the cohort (Hazard ratio: 1.29; 95% CI = 0.90-1.85). The risk of malignant brain tumors was higher in participants aged 0-6 years in the CT cohort (Hazard ratio: 3.16; 95% CI = 1.18-8.49). The female gender presented a relative risk 2.48 and 3.15 times greater of developing malignant and benign brain neoplasms in the unexposed cohort (95% CI: 1.03-5.99 and 1.17-8.45). Malignant neoplasms had a higher risk of onset within 4-5 years from the first exposure (Hazard ratio: 1.77; 95% CI: 1.07-2.92) compared to the cohort of unexposed subjects (Hazard ratio: 3.62, 95% CI: 1.47-8.91). The authors concluded that the exposure to IR from a CT scan increased the risk of developing malignant and benign neoplasms in the pediatric population.
Cross-sectional studies
Smith-Bindman et al.16 evaluated the potential risk of cancer associated with diagnostic techniques in a sample of 1119 subjects who underwent CT of the head/neck, chest, and abdomen/pelvis. The lifetime attributable risk of cancer for a head-and-neck CT was 0.23/1000 patients (range: 0.03-0.70) and for an abdominal and pelvic CT; it was four cancer cases per every 1000 patients (range: 0.83-11.1). Young women (20 years of age) had a higher risk of radiation cancer compared to women of 40-60 years.
Discussion
The medical evidence reported in this manuscript supports a relationship between IR exposure secondary to CT of the head12,15,16, neck13,16, thorax13,16, abdominopelvic muscles14,16 and hip/pelvis13,16, and a greater risk of developing a neoplastic event, even at minimal doses. Most of the documents included are retrospective cohort studies, conducted on pediatric population11-13,15,17-19 with considerable sample sizes. The main neoplasms associated with exposure to IR by performing a CT scan were leukemias and malignant brain tumors20,21 as well as meningiomas15,22.
The medical evidence found in this review describes the association between IR and solid tumors of the central nervous system and hematopoietic tumors of the myeloid line, mainly among those exposed from an early age and among females11,13,15,23-25. For example, in the pediatric population exposed to IR, a greater risk of having thyroid cancer has been described compared to adults. Likewise, women are at greater risk of developing thyroid neoplasia than men18,23. Moreover, IR is related to the risk of breast and ovarian cancer in women, but there is no clear evidence that radiation causes cancer of the breast or prostate in men23,26. In adult subjects with exposure to other carcinogenic risk factors such as smoking, obesity, and sedentary lifestyle, among others, this association may be unclear because IR would no longer be solely responsible for the damage to genetic material23,27.
Clustered DNA lesions, also called multiple damage sites, are the hallmark of the harmful effects of IR28,29. They are defined as the combination of two or more lesions, comprising strand breaks, base damage generated by oxidation, abasic sites within one or two turns of DNA helix, and created by the passage of a single radiation track29. In radiation with low linear energy transfer, such as X-rays, there may be greater damage to DNA. Therefore, the possibilities of mutation are drastically increased with exposure to IR, and they can be repaired by slow kinetics, or are left unrepaired and cause cell death or passing mitosis29,30. In the surviving cells, large deletions, translocations, and chromosomal aberrations are observed, thus inducing genomic instability, among other conditions that trigger a cascade of cellular metabolic lesions until reaching malignant behavior30. On the other hand, RI mutational signatures have been described in 319 tumors that have not received radiation, presenting a median of 201 additional deletions throughout the genome, with a size of 1-100 base pairs and without variations in cell replication time or chromatin structure. This suggests that an absolute load of mutations is necessary for the development of malignant neoplastic processes2.
The equivalent dose represents the product of the dose absorbed by a tissue and a radiation weighting factor, used to measure the effect of IR31. In medicine, the equivalent dose is expressed in millisieverts (mSv)32. The risk of cancer induced by radiation occurs at doses above 100 mSv, controversial at doses between 10 and 100 mSv and below 10 mSv, there is no data31-33. A CT of the abdomen or thoracic may have a dose of around 10 mSv, however, the repetitive performance of CT increases the mSv and the risk of developing cancer33.
Subclinical tumors that are undetectable on CT have contributed to debate in the recognition of the carcinogenic capacity of IR1,23. Multiple studies have described a time interval of 2-7 years from exposure to IR to a diagnosis of cancer, generating uncertainty about the duration of the latent phase between radiation exposure and the development of neoplastic processes15,34,35. Despite this, other variables such as the dose and frequency determine the rate of mutations and the speed of the onset of the disease1. In addition, cancer susceptibility syndromes generate a greater risk of developing a malignant neoplasm regardless of the dose or frequency of exposure to IR, which can also play a role in the relationship between IR and the risk of developing tumors2,15.
Limitations
Our study included only publications in two languages: English and Spanish. We might have missed important publications in other languages. In addition, the quality of the evidence is not evaluated in scoping reviews, because the research question is broad and encompasses a broad scope where such an evaluation would be difficult7-9.
Our review is constrained by the limitations of the included studies. One of the main limitations was the difficulty in defining the exposure and IR dose of each CT due to the different equipment used, body mass of the subjects, examination protocols, and the purpose of the examinations.
Conclusion
The studies found in this review support a relationship between CT scan-related IR exposure and increased risk of developing neoplastic events. The risk is particularly important within the pediatric population, females, and with repeated exposures. The neoplastic events that were most frequently reported were leukemias and malignant brain tumors. Due to the increase in the use of CT as the diagnostic method of choice, it is necessary to conduct a greater number of clinical studies that include a longer follow-up time, broader types of the neoplasm, and population-based subgroup analysis.
Supplementary Data
Supplementary data are available at Mexican Journal of Oncology online (DOI: 10.24875/j.gamo.22000097). These data are provided by the corresponding author and published online for the benefit of the reader. The contents of supplementary data are the sole responsibility of the authors.











 nueva página del texto (beta)
nueva página del texto (beta)