Introduction
Hypercalcemia occurs when the entry of calcium into the blood compartment is greater than the exit1. It is a relatively frequent clinical disorder and in up to 90% of cases, it is caused by hyperparathyroidism and secondary to neoplasms2. Discerning between one entity and another is not complicated since both have different behaviors3. The malignancy is often clinically evident at the same time that causes hypercalcemia. Patients with paraneoplastic hypercalcemia generally have higher calcium concentrations and more obvious signs and symptoms than people with primary hyperparathyroidism who may go years without clinical manifestations or even with slightly elevated calcium4. A known but rarely reported cause is hypercalcemia due to immobility, since it requires an exhaustive approach as it is a diagnosis of exclusion.
Immobilization in association with reduced mechanical load on the skeleton favour the imbalance between bone formation and resorption, the latter predominating by inhibiting bone formation. Thus, bone resorption with negative calcium balance leading to osteopenia, osteoporosis, and hypercalcemia that could occur from prolonged immobilization after burns, spinal injuries, major stroke, hip fracture, and bariatric surgery. Immobilized individuals with already high bone turnover, such as growing children, patients with Paget's disease, or patients with primary hyperparathyroidism or paraneoplastic hypercalcemia5, are the most frequent scenarios where hypercalcemia due to malignancy can occur.
Due to the low number of case reports in the contemporary medical literature, we consider it relevant to present the case of a patient hospitalized at the Hospital General de México who on his 90th day of hospitalization started with acute hypercalcemia. He also had low response to usual therapeutic measures. Such as hyperhydration. The investigation of the aetiology was carried out, ruling out the main causes of hypercalcemia in the hospitalized patient.
Presentation of the case
A 39-year-old male patient with a family history of a mother with rheumatoid arthritis and a father who died of an acute myocardial infarction at the age of 69 is presented. He has completed high school and previously worked as a street vendor. Among his personal pathological history, he highlighted an acute abdomen that required emergency exploratory laparotomy in this hospital with the discovery of three punctate perforations in the sigmoid sealed with an omentum and 500 mL of free purulent material in the cavity. Twenty-four hours later, he underwent emergency laparotomy due to acute abdomen with the discovery of multiple perforations in the descending colon and distal part of the transverse colon with 1000 mL of faecal material in the abdominal cavity, perforated and necrotic wall of the rectum, and fasciitis of the abdominal wall.
On days 7 and 10 after the first laparotomy, he underwent a third emergency laparotomy and emergency abdominal surgical lavage, respectively. On day 13 of admission, negative pressure therapy was applied through the open frozen abdomen. Due to complications, two more surgical scrubs were performed: an urgent exploratory laparotomy on day 23 of admission with the discovery of a hematoma of approximately 900 g, a new surgical scrub on day 27 of admission, an emergency ileostomy on day 86, and a surgical scrub on day 97 of admission. He presented diverse metabolic complications such as states of hydroelectrolyte imbalance, metabolic acidosis, and secondary septic shock.
During his first week of stay, he was diagnosed with HIV infection (Table 1). The open abdomen is staged as Bjork's grade 4, that is, complicated open abdomen, frozen, without fistula. Nutritional conditions improved and he was discharged for outpatient follow-up due to improvement. In the following two years, he presented two new episodes of sepsis of abdominal origin without requiring surgical management.
Table 1 Classification of the open abdomen of Björk 2009
| Grade | Description |
|---|---|
| 1 a | Clean without adhesions |
| 1 b | Contaminated without adhesions |
| 2 a | Clean with fixed adhesions |
| 2 b | Contaminated with fixed adhesions |
| 3 | Complicated open abdomen, with fistula in formation |
| 4 | Frozen open abdomen, with firm adhesions to the intestine, impossible to close, with or without fistula |
Twenty-five months after the onset of this first episode of acute abdomen, he was admitted again due to ileostomy dysfunction causing septic shock. He was in the intensive care unit for a long time under invasive mechanical ventilation, under sedation and analgesia. Due to failure of extubation, a tracheostomy was performed. After the general surgery, he received mixed feeding: parenteral and enteral with serial controls of serum electrolytes as well as other biomarkers. Count of 200 CD4. Cytomegalovirus and tuberculosis infections were ruled out during his stay in the intensive care unit, and antiretroviral treatment with Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide) was continued. On day 90 of this in-hospital stay, hypercalcemia was detected incidentally during routine electrolytes: albumin 2.57 mg/dL, albumin-corrected Ca 14.19 mg/dL, K 4.4 mEq/L, Mg 0.9 mg/dL, Na 130 mEq/L, P 4.7 mg/dL, creatinine 0.56 mg/dL. Due to the increase in the serum calcium, value from 8.89 mg/dL to 14.19 mg/dL in 48 hours, consultation with the endocrinology service was requested.
After a detailed anamnesis and directed physical examination, elevated serum calcium levels were corroborated to a Ph of 7.33. We request parathormone and vitamin D levels and hyperhydration management with crystalloid solutions was started.
He had no clinical evidence or imaging studies previously performed since his stay in the intensive care unit due to the previously mentioned multiple complications. He had simple and contrast-enhanced tomographies of the abdomen, thorax, and pelvis, with no data compatible with solid organ malignancy, haematological malignancy. or granulomatous diseases such as tuberculosis.
Due to the history of cytomegalovirus (CMV) infection that caused intestinal perforation, we discarded cytomegalovirus infection on this admission.
When collecting the results of serum levels of intact parathormone (PTHi) and 25 hydroxyvitamin D, hypercalcemia with mild hypophosphatemia was concluded, with PTHi and 25 hydroxyvitamin D at 4.1 pg/mL and 5.0 ng/mL, respectively.
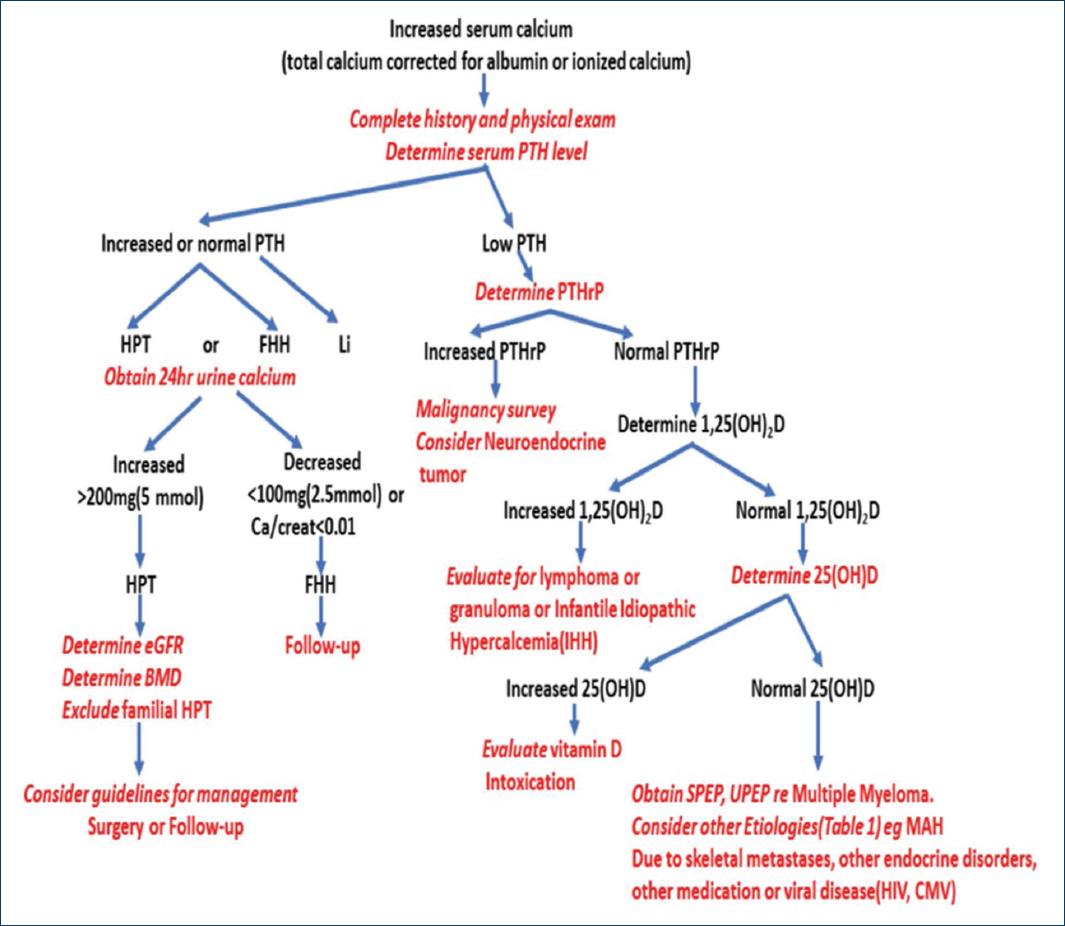
According to the hypercalcemia diagnostic algorithm (Fig. 1), hypercalcemia was corroborated with low iPTH and without clinical or imaging evidence of malignancy and with low vitamin D, we considered ruling out multiple myeloma since no cytopenias or blastic lesions were found in the examined bone structures, nor bone pain, neither renal involvement, nor granulomatous diseases, hyperthyroidism and bone neoplasms. Hypercalcemia due to immobilization was diagnosed due to a history of being in bed for at least 3 months complicated by clinical malnutrition, recovery due to an open frozen abdomen for 28 months, drastically limiting his mobilization until absolute rest in the last 90 days.
Due to the progressive increase in hypercalcemia and hypophosphatemia despite management with hyperhydration, zoledronic acid 4 mg was administered in a single dose with a decrease after 48 hours and normalization of calcium on the fourth day after having administered this bisphosphonate, obtaining a favourable response, something unlikely if it were paraneoplastic hypercalcemia (Table 2).
Table 2 Chronological record of laboratory results used in the diagnostic approach of this case
| Laboratory results relevant to the clinical case | |||||
|---|---|---|---|---|---|
| Date | Albumin-corrected calcium (mg/dL) | Phosphorus (mg/dL) | PTHi (pg/mL) | 25 OH vitamin D (ng/mL) | Observations |
| 21/05/21 | 8.89 | 6.2 | |||
| 24/05/21 | 14.19 | 4.4 | Onset of hyperhydration | ||
| 26/05/21 | 14.95 | 3.1 | 4,1 | 5.95 | Zoledronic acid 4 mg/5mL |
| 27/05/21 | 16.93 | 3.3 | |||
| 28/05/21 | 17.79 | 3.2 | |||
| 29/05/21 | 12.73 | 2.3 | Low calcium | ||
| 31/05/21 | 10.02 | 1.3 | |||
| 01/06/21 | 9.38 | 1.3 | |||
Discussion
In the approach to hypercalcemia, there are causes dependent on parathyroid hormone and causes not associated with parathyroid hormone, so a first step is to determine the iPTH concentration as presented in this case. Within this last category are hypercalcemia due to increased bone resorption, highlighting that of paraneoplastic origin, presenting in up to 20 to 30% of cancer patients5 and it is the most common cause of hypercalcemia in hospitalized patients. Neoplastic disease is usually clinically evident at the time hypercalcemia manifests, and is generally associated with a poor prognosis6. This diagnostic possibility can be approached with the serum determination of the peptide related to parathormone (PTH-rP). However, in our environment it is not easily accessible, so through the systematic review of the clinical evolution and the paraclinical studies carried out during at 28 months. This possibility was displaced to a less probable cause, as thyrotoxicosis, renal failure, increased calcium intake, hypervitaminosis D, prescription drugs such as lithium, thiazides, calcium carbonate or calcium citrate, and other diseases such as pheochromocytoma and adrenal insufficiency, acute kidney injury and systemic lupus erythematosus7, leaving hypercalcemia due to immobility as a probable etiology.
Hypercalcemia due to prolonged immobility has been described mainly in children with orthopedic immobilization after complex fractures. Immobilization leads to increased bone resorption, causing increased serum and urinary calcium levels8. A study of 46 pediatric individuals immobilized for ischemic necrosis of the femoral head demonstrated a transient increase in urinary calcium without an increase in serum calcium after the first week of immobilization. There were no alterations in the normal levels of PTH and vitamin D. It was concluded that hypercalcemia due to immobilization occurs in isolation in patients with probable conditions of high bone turnover9.
There is evidence of increased resorption in patients immobilized for a long time after suffering burns, spinal cord injury, major stroke, hip fracture, and bariatric surgery. These data were obtained by studies of bone mineral density in patients with hospital stays with said conditions. Negative calcium balance occurs as a result of urinary calcium loss and can last for several months8. One study found that up to 0.5% of bone mass can be lost during each month of immobilization, leading to osteopenia and osteoporosis10.
The main determinant in the temporality of the appearance of hypercalcemia due to immobilization is the renal function. The greater the deterioration of renal function, the faster hypercalcemia becomes evident11, although it is not a determining factor since it has been documented that hypercalciuria precedes hypercalcemia due to immobility in young adults with spinal cord injury and can give rise to nephrocalcinosis; this being the one that can lead to deterioration of renal function in young patients without previously known renal disease12.
In this particular case, renal function was preserved at the time of the first determination of hypercalcemia, despite the multiple complications it had presented. Immobilization hypercalcemia is caused by increased calcium efflux from bone due to increased osteoclastic activity. This results in increased serum calcium, reduced parathyroid hormone (PTH) and vitamin D 1,25(OH)2 levels associated with elevated urinary calcium and N-telopeptide levels. In immobilization hypercalcemia, low PTH levels are seen due to the inhibitory effect of high calcium levels on PTH secretion. Low levels of vitamin D 1,25(OH)2 are observed because PTH is required to convert vitamin D 25(OH) to vitamin D 1,25(OH)213. Immobilization activates remodeling loci and decrease of the osteoblastic stimulus, which leads directly to a local reduction in bone mass, the greater activation multiplying the effect of the deficit in bone formation. Although the precise mechanism remains to be defined, loss of mechanical stress has been shown to be relevant to bone wear, and attenuated parathyroid function and biosynthesis have been considered to be associated with immobilization causing accelerated bone resorption14.
In this context, the diagnosis of hypercalcemia due to immobility was confirmed due to a stay in this hospital of at least 90 days derived from the Bjork 4 frozen abdomen. The diagnosis of hypercalcemia due to immobilization requires a thorough evaluation. In the case of our patient, the challenge was greater since he had an underlying disease that suggests more probable etiologies of hypercalcemia such as cancer, lymphoma, active CMV infection, tuberculosis, etc.
The recommended treatment is hydration with crystalloid solutions and the concomitant use of bisphosphonates with an action time of 2 to 3 days with a decrease in serum calcium. The favorable clinical response to a single dose of zoledronic acid is not the usual response in the case of neoplastic processes, the main differential diagnosis15. During the following 6 weeks of hospitalization, the patient did not present hypercalcemia again.
There are no reports of other cases of hypercalcemia due to postoperative immobility in the previous two decades. The last one was in 2002 and corresponds to two patients who underwent gastric banding with post-surgical complications who developed hypercalcemia after a prolonged hospital stay12, another scenario different from the one usually reported, hence the relevance of presenting this case report.











 nueva página del texto (beta)
nueva página del texto (beta)