Introduction
Neuroenteric cysts (NECs) are congenital malformations of endodermal origin, denoting 0.01% of all tumors and 16% of cystic lesions of the central nervous system, as well as 0.15-0.35% of intracranial tumors. Most of these lesions have a spinal location, with symptoms related to the size and location1. Conversely, Klippel-Feil syndrome (KFS) is defined as the fusion of two or more cervical vertebrae, with a classic triad of low hair implantation, short neck (brevicollis), and limitation of cervical movements2. Uncommonly, KFS has been associated with intracranial tumors; most frequently are dermoid or epidermoid cysts3, a relationship previously reported by Pai et al. (2007) by describing 3 cases4. The relationship between NEC and osseous defects such as spina bifida, split cord malformation, and Klippel-Feil syndrome has been described5. However, the relationship between the intracranial location of a NEC and KFS has not been previously reported. Therefore, the origin of these disorders has not been exactly determined, for which we present a case was reported of a 21-year-old Mexican male with KFS and a symptomatic posterior fossa located NEC that was removed through a midline suboccipital craniectomy for posterior approach, to describe the characteristics of the patient, as well as to unify several theories to establish the possible nature of the association between these two entities.
Case presentation
A 21-year-old male patient presented to the hospital without relevant history, began with occipital headache of 1 month of evolution aggravated by Valsalva maneuvers, associated with nausea and emesis. Physical examination showed low hair implantation, brevicollis with restricted range of motion, papilledema, dysdiadochokinesia, and right dysmetria. Head computed tomography (CT) exhibited a heterogeneous cystic lesion on the posterior fossa causing obstructive hydrocephalus, for which an urgent high pressure left precoronal ventriculoperitoneal shunt was placed, demonstrating clear cerebrospinal fluid. Afterward, brain magnetic resonance imaging (MRI) was performed, finding an infratentorial lesion, dorsal to the right cerebellar hemisphere, ovoid shape, with regular and defined borders, composed of a nodular portion in contact with pia mater, and multiple punctate flow absences, isointense on T1, heterogeneously hyperintense on T2 and Fluid attenuated inversion recovery (FLAIR) sequences, with diffusion restriction on its central portion and contrast enhancement, whose measurements were 17 × 14 × 15 mm in major axes, another remaining cystic portion was hyperintense on T1, hypointense on T2, FLAIR, and Apparent diffusion coefficient, without diffusion restriction or contrast-enhancement, whose measurements were 46 × 49 × 46 mm (Fig. 1A,B), Cerebral angiography (CAG) was negative for vascular alterations (Fig. 1C,D) and spectroscopy displayed increased N-Acetyl-Aspartate and choline peaks (Fig. 1E). Anteroposterior and lateral static and dynamic cervical spine radiographs (XR) and spine CT exposed lordosis rectification, flexion, and extension limitation, C2-C3 and C4-C5 anterior and posterior elements fusion, and spinal MRI exhibited vertebral bodies fusion defects on the anterior portion of these structures, without thoracic, lumbar, or sacral alterations (Fig. 2).
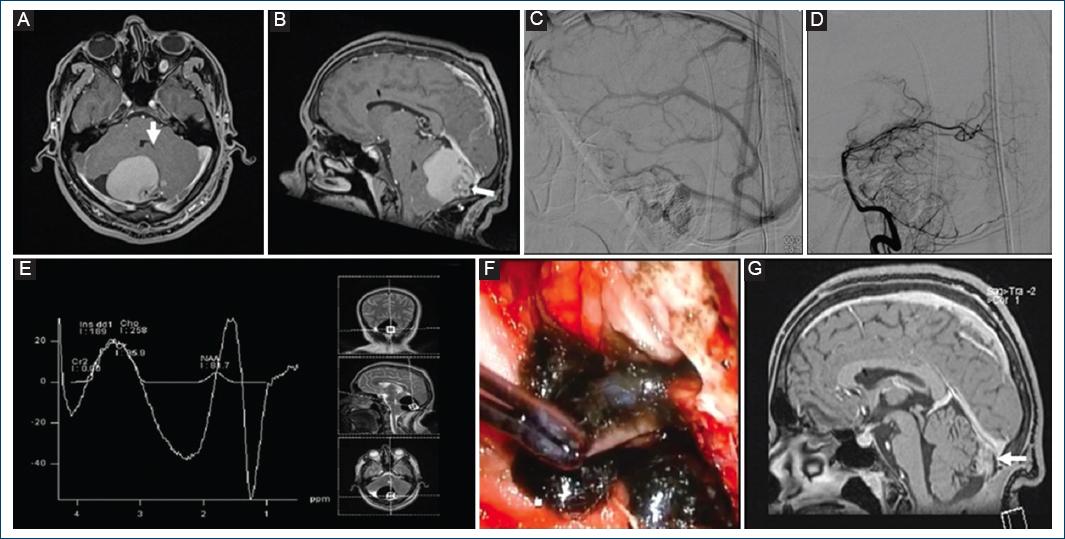
Figure 1 A and B: pre-surgical brain contrast-enhanced T1-weighted magnetic resonance imaging (MRI) with evidence of an extra-axial lesion (white arrow) in the posterior fossa (A: axial section. B: sagittal section). C and D: cerebral angiography without alterations (C: late venous phase. D: early arterial phase). E: spectroscopy without choline elevation. F: intraoperative image: Tumor capsule with a good cleavage plane, and liquid content of oily material inside the capsule. G: post-surgical MRI sagittal section showing a small residual lesion (white arrow).
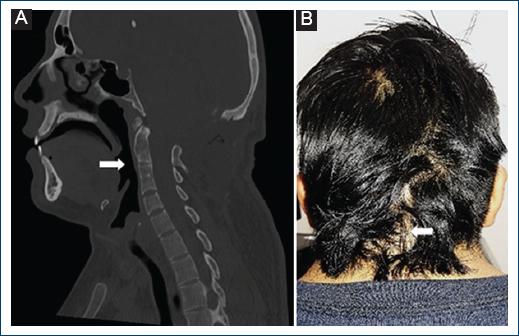
Figure 2 A: cervical spine computed tomography (CT) with evidence of C2-C3 and C4-C5 anterior elements fusion (white arrow). B: post-surgical patient photograph depicting low hair implantation and short neck (brevicollis) from Klippel-Feil Syndrome, and mature surgical scar at follow-up (white arrow).
Due to data related with KFS was clinically diagnosed. Normal echocardiography, simple and contrasted thoracoabdominal CT, and renal function tests were obtained; otorhinolaryngology assessment cursed without hearing alterations, confirmed the syndromic diagnosis. Subsequently, a midline suboccipital craniectomy, resection of C1 posterior arch and tumor excision was performed, obtaining a cystic lesion with a mural nodule at the inferolateral right torcular herophili level, with leakage of greenish fluid (Fig. 1F). Complete resection of the capsule was achieved, with a histopathological study that reported smooth, opaque, light gray color walls, with tortuous vessels, and peripheral solid, anfractuous, gray-green areas of firm consistency, clear brown content, and soft consistency show a characteristic image of NEC, positive to alcian blue and negative to periodic acid Schiff stains (Fig. 3). The patient had a favorable clinical evolution, receiving medical discharge to home after 3 weeks, with adequate follow-up 9 months after surgery, identifying by MRI a residual nodular image adhered to the straight sinus (Fig. 1G).
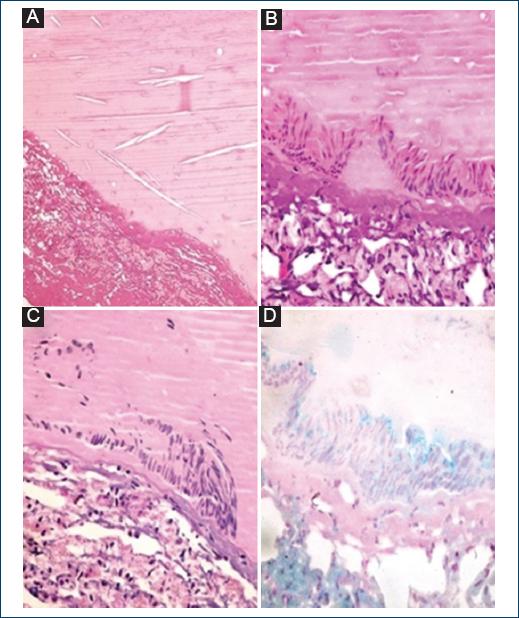
Figure 3 Histopathology images. A: hematoxylin and eosin staining (H&E) (40×); cyst wall and proteinaceous content with some spaces for cholesterol crystals. B: H&E (400×); detail of the columnar epithelium. C: periodic acid Schiff staining (400×); detail of staining negative epithelium. D: alcian blue staining (400×); detail of the epithelium with cytoplasmic positivity.
Discussion
This case suggests a possible association of genetic origin between KFS and NEC. KFS can be subclassified into three morphological subtypes of KFS. Type I involves a single congenitally fused cervical segment; Type II includes multiple non-contiguous, congenitally fused segments, and Type III comprises multiple contiguous, congenitally fused cervical segments, with Type II being the most common. Because this patient presented with a C2-C3 and a C4-C5 fusion, the KFS classification correlates with a Type II, commonly associated with mutations in the MEOX1 gene located on chromosome 17q21.31. Other commonly mentioned genetic alterations are mutations in the GDF6, GDF3, and MY018B genes2,6. On the other hand, NEC is commonly related to other neoplasms such as cystic teratoma and teratoma, and the gene associated is KRAS (KRAS Proto-Oncogene, GTPase), a gene related to pathways involved in development and cytoskeletal signaling1. Therefore, the genetic association between KRAS and KFS Type II genes (MEOX1, GDF6, GDF3, and MY018B) is not clear. Alazami et al. (2014) described a patient with KFS with a mutation in the MYO18B gene, where at the time of KRAS gene sequencing, it showed no abnormalities. However, this patient did not have the presence of intracranial NEC7. Although there is no genetic point mutation that explains the association between these two diseases, KFS could be related to the failure of the formation, segmentation and ordering of cervical sclerotomes in the formation of encephalic flexures, and NEC is caused by abnormal endodermal-ectodermal adhesion during gastrulation, with persistence of endodermal elements near the notochord in the neuroaxis, this alterations in embryological development of the brainstem and spinal cord relative to segmentation/gastrulation and the craniocaudal development in relation to the neuroaxis with persistence of embryological structures could explain the coexistence of these two entities. Unfortunately, it was not possible to make a genetic diagnosis (identification of an altered gene) due to the socioeconomic context of the patient, and loss of follow-up. KFS patients with characteristics similar to those of the patient described in this report usually have a mutation in the gene MEOX12. Furthermore, some data support the relationship between two entities; KFS has been associated with other types of intracranial tumors such as teratomas and epidermoid/dermoid cysts3. Dermoid and epidermoid cysts are usually more lobulated, in contrast to the rounded appearance of NEC. In addition, they are more likely to calcifications; a key factor to differentiate them is the irregular contours of the dermoid cyst8.
Cervical spine XR show scoliosis, vertebral fusion, and instability; spinal CT with three-dimensional reconstruction is useful in surgical planning to reveal unsegmented unilateral rods or cervical stenosis; spinal MRI is indicated in neurological deficits to reveal spinal compression, stenosis, and syringomyelia. The Samartzis classification divides the disorder into three types with high prognostic value. Treatment is based on the detection and management of associated systemic alterations. Forty-three percent of patients will require decompression and spinal stabilization in the second or third decade of life, depending on high-risk patterns such as C2-C3 fusion with occipitocervical synostosis, extensive cervical fusion with abnormal occipitocervical junction, and two fused cervical segments separated by an open joint space. Our patient did not require surgical management of this malformation due to the lack of clinical repercussion2.
NEC was described in 1928, the first histologically confirmed case was reported in 1934, historically using various designations, and the first intracranial NEC was informed in 1962, with more than 140 cases reported since then. Frequency is higher in men, although recent series have found a female predominance, and presentation ranges from the neonatal period to 71 years, with initial intracranial finding in the second or third decades of life. About 90% of intracranial lesions are found on the posterior fossa, specifically at prepontine and prebulbar cisterns, cisterna magna, cerebellopontine angle, fourth ventricle, and dorsal to the cerebellum. The etiopathogenesis is due to abnormal endodermal-ectodermal adhesion during gastrulation, with persistence of endodermal elements near the notochord in the neuroaxis, which would explain the association to spina bifida, diastematomyelia, and vertebral body alterations by complete persistence of the neuroenteric canal, while the partial form causes an isolated intraspinal or intracranial cyst. Supratentorial localizations are even more exceptional, caused by Seessel’s pouch persistence. Infratentorial lesions present headache, nausea, emesis, and cranial nerve alterations such as vertigo, tinnitus, hypoesthesia, or trigeminal neuralgia, among others. Its existence can be suspected in recurrent meningitis due to a fistula to the aerodigestive tract that causes slow growth because of active secretion from epithelial cells, cerebrospinal fluid osmotic retention, or hemorrhage, with an unknown transient regression mechanism involving intermittent compression of neural structures, spontaneous leakage, and recurrent accumulation, in addition to melanin production and subsequent inflammation. Few articles refer to its debut with hydrocephalus. Accompanying disorders are cutaneous abnormalities and intestinal malformations, rare in correlation with intracranial lesions. Clinical manifestations can be acute or insidious, with a course ranging from 4 months to 39 years9.
In head CT, NEC is hypodense lesions without contrast enhancement, although the density varies depending on their protein concentration. On MRI, they appear heterogeneous, but are generally well-demarcated, extra-axial, rounded, ovoid, or lobulated cysts, hyperintense on T1, T2, and FLAIR, without contrast-enhancement, with slight diffusion restriction in diffusion-weighted imaging (DWI) due to xanthogranulomatous changes or presence of melanin, hemosiderin, proteins, mucopolysaccharides, and cholesterol; occasionally, they can be hypointense on T1 and T2 due to increased protein content or bleeding, which can be ruled out on susceptibility-weighted imaging (SWI) by hypointensity. They do not produce edema and are avascular lesions on CAG. Ventral to the brainstem and vertebrobasilar arterial displacement on MRI can be pathognomonic, related to vascular mesodermal origin and extrapial disposition. Radiological differential diagnoses on the posterior fossa are arachnoid cysts, metastases, classic or atypical epidermoid cyst (“white epidermoid”), dermoid cyst, cholesteatoma, ependymoma, schwannoma, neurocysticercosis, hemangioblastoma, and pilocytic astrocytoma, where only 23-28.5% can be correctly diagnosed preoperatively10.
Macroscopically, they are yellow, milky white, gray, or red cysts, with thin walls similar to arachnoid, and transparent, mucoid or xanthochromic liquid content, unusually blood, pus, calcifications, or keratinized debris adhering to the adjacent pia mater. Histopathological studies reveal benign lesions with simple, pseudostratified, cuboid, or columnar epithelium walls, and collagenous fibrous connective tissue basal membrane, lined with gastrointestinal or ciliated respiratory epithelium, sporadically mixed or with pancreatic epithelium and squamous metaplasia, and the presence of mucin-secreting goblet cells reactive to alcian blue and periodic acid–Schiff stains. In immunohistochemistry, they are positive for cytokeratin, epithelial membrane antigen, and carcinoembryonic antigen. Degeneration to adenocarcinoma is extremely unusual and only occurs in intracranial locations (nine patients reported). In general, carbohydrate antigen 19-9 is positive, while elevated MIB-1 labeling index suggests malignancy. The Wilkins and Odom histological classification divides the lesions into three types, but it is only applicable to spinal cysts. However, no correlation between imaging findings and pathology has been found in systematic reviews11.
Surgical resection is the first-line treatment for NEC with the objective of complete total resection. However, vertebral anomalies such as KFS or extensive adhesion to neuronal anatomy can make complete resection difficult and dangerous. Current literature lacks a consensus on the most appropriate surgical approach, where probably, the posterior technique is associated with fewer intraoperative complications. Risk related to this approach is primarily linked to the typical complications associated with laminectomies such as nerve root, spinal cord, and dural injury. In posterior resection, the aspiration of cyst contents may carry a chance of membrane rupture, causing cyst leakage leading to meningitis1. Reports of after-surgery recurrence have fluctuated between 0% and 37%. Kim, et al. observed no recurrence in their series of eight patients12. Holmes et al. (1978) observed recurrence in only 4% of patients13. Chavda et al. (1985) published the longest follow-up at 30 years and observed a 37% recurrence rate. Highlighting that all cases of recurrence in their study were seen in patients who underwent partial surgical resection13. Therefore, partial resection remains the primary risk factor for recurrence in NEC. In contrast with our patient, where despite a complete resection, a recurrence of the tumor mass occurred.
It seems that the relevance of this manuscript is in describing the relationship of these two clinical entities. However, this requires a deeper study of the genes involved through molecular tests (which could not be performed in this patient due to loss of follow-up). The true importance of this report lies in its practical applicability for the neurosurgeon, emphasizing that any patient with KFS who presents a cystic lesion in the posterior fossa, a complete resection should be sought because it could be dealing with a NEC, a lesion with a high rate of recurrence.
Conclusion
The genetic association between NEC and KFS is unclear. However, these two entities are related to alterations in the embryological development of the brainstem and spinal cord with the persistence of embryological structures, which is why the coexistence of these two pathologies is justified. Surgical resection is the first-line treatment for NEC with the goal of complete resection. However, vertebral abnormalities such as those seen in a patient with KFS can make complete resection difficult and dangerous, where partial resection remains the main risk factor for recurrence. Therefore, complete resection is crucial for the prognosis in this type of patients.











 nueva página del texto (beta)
nueva página del texto (beta)


