Introduction
Sarcomas are a rare and heterogeneous group of neoplasms of mesenchymal origin1. The clinical presentation of sarcoma is very varied. The main symptoms are rapid and gradual growth tumor that can be associated with pain in 50%; the pain is related to the compression effect2,3.
The acellular dermal matrix (IntegraÒ) is composed of a laminated upper layer of Silastic, resembling epidermis, it is sufficient to control water loss and prevent the invasion of microbes. The lower layer has a highly porous structure and is composed of cross-linked coprecipitate of bovine collagen and chondroitin 6-sulfate, which is derived from shark cartilage2,3. In addition, the pore size design (20 to 125 um) of the dermal layer allows migration of endothelial cells and fibroblasts from the patient into the matrix2. Both layers serve as a matrix for the migration of fibroblasts, macrophages, lymphocytes, and capillaries derived from the wound3,4.
Case report
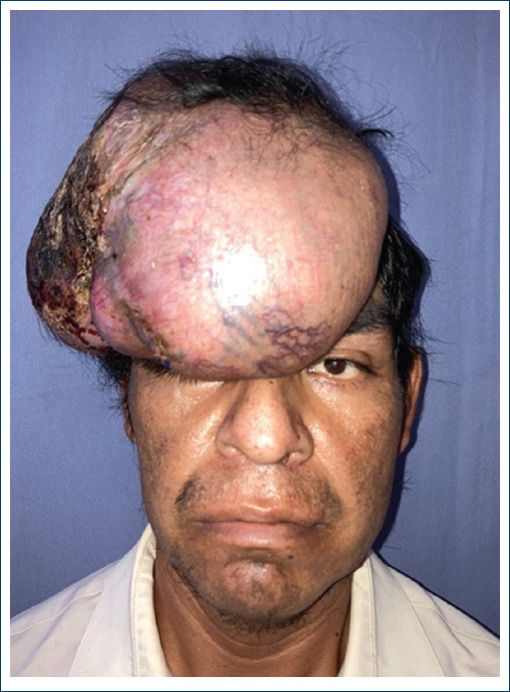
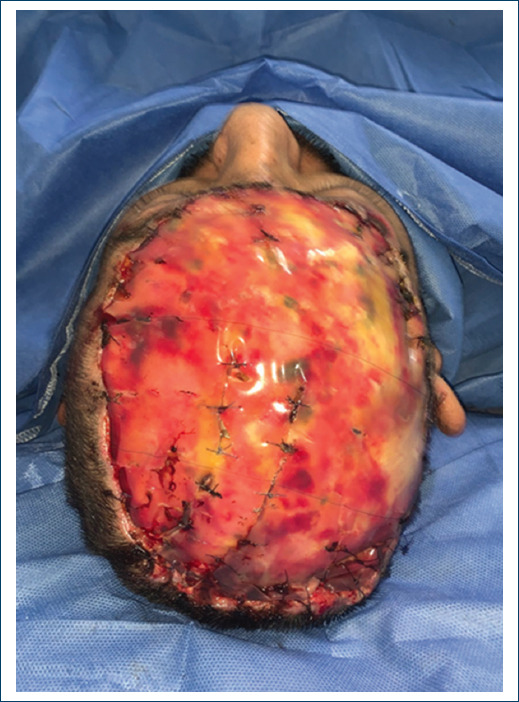
The patient was a 45-year-old male, with not chronic-degenerative diseases. His condition began in January 2021, with growth of a temporary scalp tumor, with abrupt growth until it involved the frontal, parietal, and right occipital surface of approximately 35 x 28 x 25 cm, without compromise of adjacent organs and no therapy. Biopsy reports a high grade soft tissue sarcoma. A resection of the tumor was performed by the surgical oncology unit, with the final result of the surgical piece measuring 30 x 26 x 16.5 cm, it was a histological type of fibrosarcoma with periosteal exposure, performing external table fenestrations. A new management of Skin coverage to use and cover with dermal matrix, developing a complete neodermis after 21 days of regeneration, with review every 7 days was performed. As final skin coverage, a partial thickness graft is placed, achieving 100% integration (Figs. 1-4).
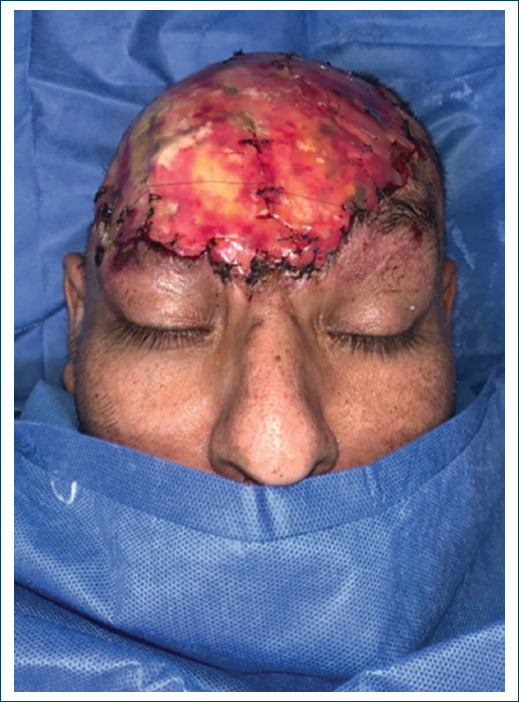
Figure 4 Placement of Acellular Dermal Matrix - Integra, in its second replacement, front view, covering with mepilex AG.
During the follow-up of the patient, with this innovative technique in patients with neoplasms, we done replacement of advanced absorbable silver dressings (Mepilex AG) on 3 occasions (every 7 days until day 21) prior to the last application, with application of graft (Figs. 5 and 6).
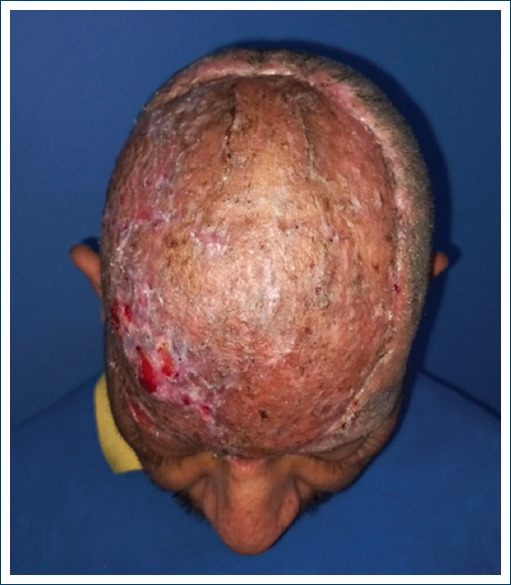
At day 21, the neodermis is assessed, the silicone layer of the dermal matrix is removed, achieving a 100% neodermis, partial thickness grafts of the Ollier - Thiersch type are taken, meshing it, proceeding to fixation of the graft covering the neodermis and the full thickness of the bloody area, which is covered with perforated silicone (mepitel One) and clip with advanced absorbable silver dressings (mepilex AG) achieving complete coverage and fixation of the grafted area. It is discovered after 7 days, assessing whether there is any complication and a minimum of exudate is observed (Figs. 7 and 8).
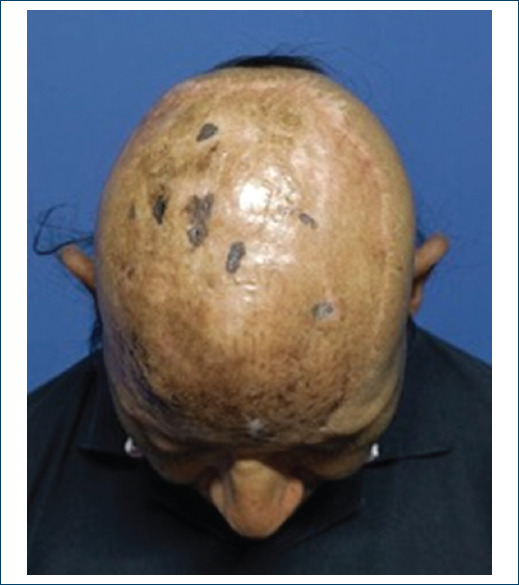
We proceed to remove the perforated silicone dressing; which, is maintained with adequate exudate, without compromise, observing a 100% integration with this new method applied and algorithm designed for the management in these patients. Therefore, at the follow-up for 1 month, complete and adequate integration is observed evolution, currently under follow-up by oncology to perform radiotherapy (Figs. 8-10).
Discussion
Sarcomas represent 1% of malignant tumors in adults2, affecting only 1 to 2 of every 500,000 people worldwide, with approximately 10,000 new cases per year in Mexico3. The mean age of patients with soft tissue sarcoma is 53.45 years for women and 55 years for men4.
There are more than 70 histological types of sarcomas which vary in biological and clinical characteristics4. In general, they can be divided according to their origin, into soft tissue sarcomas and bone sarcomas5. The most common subtypes are pleomorphic sarcoma, gastrointestinal stromal tumor, liposarcoma, and leiomyosarcoma. The most common primary sites are extremities (43%), trunk (10%), viscera (19%), retroperitoneum (15%), and head and neck (9%). Generally, the most frequent site of metastasis is the lung, however, the affected area of metastasis depends to a great extent on the site where the primary tumor originated4,5.
Approximately 90% of cases occur sporadically; in postpartum women there is a risk of genetic mutations (such as Li-Fraumeni syndrome, Neurofibromatosis of von Recklinghausen disease); as well as environmental risk factors such as ionizing radiation, radiotherapy or chemical exhibitors4,5.
The clinical presentation of sarcoma is indistinct, because it presents a rapid and gradual tumor growth, which can cause pain. The size of the tumor can develop compression of attached structures and exacerbate pain5.
Due to its low incidence and heterogeneity, the diagnosis is complicated and delayed, which directly affects the results of the assigned treatment. The presence of a tumor in the soft tissues should be treated by a specialized multidisciplinary team within referral centers as soon as a diagnosis of sarcoma is suspected6,7. Some essential elements for diagnosis are a complete clinical history, an adequate physical examination, imaging studies of the primary tumor and the identification of metastases, and biopsy (punch, incisional, or excisional)6.
The treatment serves as a new therapeutic option, as well as a new alternative to the use of dermal matrices, when this combined with bloody areas due to neoplasia it improves survival. The primary strategy is based solely on surgical resection, although it has been shown that by adding radiotherapy can increase success rates. There are several approaches in which surgical resection can be managed: it can be marginal, extensive or radical, and the choice is made based on the level of malignancy previously analyzed. In many cases, especially when it comes to sarcomas in the extremities, surgeries with greater extension of margins are reserved for the last line of treatment because it is not only the resection of the tumor that matters, but also the function and quality of life of the patient6. Radiotherapy and chemotherapy (with the anthracycline-based regimen being the first line of treatment, followed by Gemcitabine and Pazopanib6-based treatments) can be used as forms of neoadjuvant treatment, for palliative purposes in case of severe metastases, or as control local growth7.
In 1981, Burke and Yannas introduced the dermal regeneration template, it is an acellular matrix composed of two skin-like layers, which has currently been promoted as a use for survival in patients with multiple comorbidities. Current protocols (Fig. 11) describe how to use it with oncological and trauma management8.
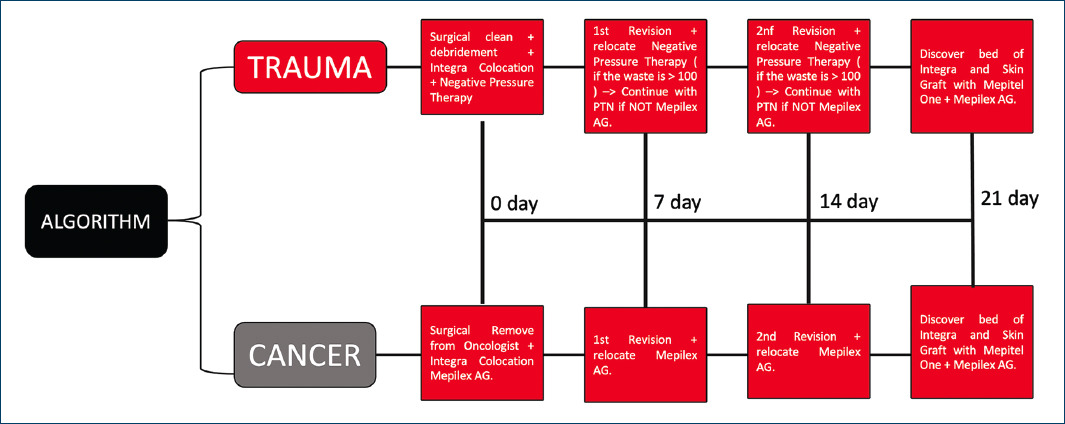
Figure 11 Standardized management of dermal matrix placement (Integra®). Explains how the dermal matrix is used in a new way to implement reconstruction due to cancer or trauma, according to a new established management.
The top layer of the Silastic sheet is an epidermis-like structure, which is sufficient to control water loss and prevent microbial invasion. The lower layer has a highly porous structure and is composed of a cross-linked coprecipitate of bovine collagen and chondroitin 6-sulfate, which is derived from shark cartilage9. In addition, the pore size design (20 to 125 um) of the dermal layer allows migration of endothelial cells and fibroblasts from the patient into the matrix9. Both layers serve as a matrix for the migration of fibroblasts, macrophages, lymphocytes, and capillaries derived from the wound9,10.
The migration of these cells will replace this layer with the patient’s own fibroblasts, a collagen network for the synthetic tissue to subsequently degrade. The matrix leads to the generation of a neodermis that is histologically very close to normal human dermis. The synthetic polysiloxane (silicone) polymer layer is a temporary layer that provides wound closure, relieves metabolic stress due to fluid and electrolyte losses, provides a barrier against microorganisms, and delays the need for autograft10.
Initially, acellular dermal matrix was approved for use in acute burn injuries, but it has been used in a variety of pathologies including full-thickness acute burn wounds, burn scars, and the treatment of purpura fulminans10. Today, it is widely used in full-body reconstructive procedures, on painful or contracted scars, skin resurfacing, and coverage of full-thickness skin graft donor sites, however, this item has the tendency to develop and describe how this algorithm (Fig. 11) can be the best option as a surgical alternative for the management of bloody areas due to cancer and trauma11.
The standard use of acellular dermal matrix reconstruction requires two steps: it forms a neodermis through ingrowth of the host’s vessels, followed by the application of a thin, split-thickness skin graft at a later date of 14 to 21 days, in a current and innovative way that improves and describes the algorithm (Fig. 11)12.
There is a tendency to explore various ways of implementing single-stage acellular dermal matrix reconstructions where the matrix and graft are placed at the same time, but more research is needed. In general, the authors are of the opinion that a stepwise procedure (Fig. 11) should be used when possible, as it provides effective and durable results for all defect sizes13.
Conclusion
Soft tissue sarcomas are malignantly distributed with rapid and gradual growth, showing clinical exacerbation depending on the tumor stage at the time of diagnosis. Timely innovative treatment, as in our case, can show an improvement in survival. A dermal matrix shows a new way to stimulate survival and clinical improvement without morbidity.
The case presented is a clear example of how to stimulate survival in patients with large-volume tumors involving skin and soft tissues. The management of an extensive bloody area in the scalp due to cancer gives us the initial guideline to establish a management algorithm in this type of patients (Fig. 11). Within our surgical practice, microsurgical flaps for full thickness coverage, such as the free-muscular latissimus dorsi flap or the omentum flap for this wide coverage, are viable options. However, in our patient, who due to the strong history of a low risk of survival (30 to 45% at 5 years) due to conclusive histological lineage, in addition to blood sequestration, this innovative treatment was a viable option.
This is a clinical case that shows an adequate multidisciplinary management and a medium-term survival which can be increased with the initiation of radiotherapy.











 nueva página del texto (beta)
nueva página del texto (beta)