Introduction
In 2020, prostate cancer ranked fourth in the world in terms of incidence among all malignant neoplasms, with 1,414,259 cases and a rate of 30.7 cases per 100,000 inhabitants, the absolute number of deaths in the world reported in the same year was 466,003 patients with an adjusted mortality rate of 7.7. In Mexico, the rate was 10.6, with 90,222 deaths; the incidence was 195,499 cases with a rate of 42.241, which represents a public health problem in our country. Prostate cancer has particular biological features compared to other types of malignant tumours. Its growth is slow and clinically silent, sometimes not very aggressive, which makes it a tumour with a high prevalence and relatively low mortality. In radiation therapy, we classify them into low, intermediate and high-risk groups based on three parameters: prostate-specific antigen level, Gleason score and extent of disease in the pelvis. Histologically speaking, more than 90% of prostate malignant tumours correspond to acinar adenocarcinomas, the vast majority originate from the peripheral zone of the prostate and generally have an orderly behaviour. The main site of metastasis is the bone with a predilection for the axial skeleton.
An adequate screening programme, combined with safe and effective treatment, allows patients to enjoy an excellent local control rate and a good quality of life. The demand for radiotherapy services for prostate cancer has increased significantly, in parallel with technological advances. Radiotherapy has proven to be a safe and effective treatment for prostate cancer.
Background
Radiotherapy has long been the mainstay of radical treatment for prostate cancer patients, with results comparable to those of radical prostatectomy, but with less morbidity. Pioneering radiotherapy studies2 gave doses of 64-70 Gy in conventional fractionation (1.8-2 Gy per session) with conflicting results and high biochemical failure rates. Subsequently, dose escalation studies3 were initiated, with favourable results and high local control rates, but with increased acute and chronic toxicity due to the use of three-dimensional (3D) conformal technology. The main toxicity of radiotherapy is in the bladder (cystitis) and rectum (proctitis) and we classify it as acute and chronic depending on the time of onset. In general, acute toxicity is considered to be that which occurs during treatment and up to 6 months after the end of treatment, and chronic toxicity occurs 6 months after the end of treatment. Thus, we have acute and chronic post-radiation cystitis and post-radiation proctitis. There are different scales for assessing toxicity, the main one being that of the Radiation Oncology Education Collaborative Study Group in the US and Europe. Currently, according to international guidelines4, the standard treatment requires doses above 78 Gy in conventional fractionation, with sessions from Monday to Friday for 8 weeks. Technological advances in intensity-modulated radiation therapy (IMRT) and its dynamic variants such as volumetric intensity-modulated arc therapy (VMAT), as well as image-guided radiation therapy (IGRT), have made it possible to offer more precise treatments with less morbidity. Because the number of prostate tumour cells have decreased5, studies have been initiated over the past decade to evaluate the efficacy and safety of moderate hypofractionation (doses of 2.5-4 Gy per session), and we now have mature data indicating that moderate hypofractionation is equivalent to conventional fractionation, with the advantage that it can be administered in 5 weeks or less. Meta-analysis studies6 show even better biochemical and clinical control rates, with conflicting results for acute toxicity, but with less chronic toxicity compared to conventional fractionation. Maximum androgen blockade is currently used in intermediate and high risk tumours and consists of the complete suppression of testosterone production (biochemical castration); this treatment is not considered a radical treatment, so it is not sufficient to treat patients with this treatment modality alone; it is a co-adjuvant treatment to radical treatments (surgery or radiotherapy).
Since 1 January 2017, the radiotherapy department of the Hospital Regional de Alta Especialidad (HRAE) "Centenario de la Revolución Mexicana" ISSSTE Morelos began the protocol of moderate hypofractionation in prostate cancer, administering total doses of 70. 2 Gy in 26 fractions, 2.7 Gy per fraction with VMAT, IGRT and concomitant incremental technique for elective pelvic lymph node irradiation, based on the results and fractionation of Pollack7, which was performed in the Anglo-Saxon population. This is the first report with such a treatment scheme in a Mexican population.
Objective
To describe the institutional experience of treatment with moderate hypofractionation administered with intensity-modulated volumetric arc therapy in localised prostate cancer in the Mexican population of the HRAE "Centenario de la Revolución Mexicana", Morelos.
Material and methods
A retrospective analysis was performed on all patients diagnosed with prostate cancer at the HRAE from 1 January 2017 to 31 January 2021 treated with the radical-intent radiotherapy. The treatment plan was done according to the IMRT - VMAT modality and was administered on an Elekta linear accelerator with "Agility" multi-leaf collimator. 70.2 Gy were prescribed to the prostate and seminal vesicles (2. 7 Gy per session) with elective irradiation of 50 Gy to the pelvic lymph nodes according to the treating physician’s criteria using Roach’s formulas8 to calculate the probability of lymph node involvement. A risk greater than or equal to 15% was considered significant. Both prescriptions were given with a concomitant augmentation technique in 26 sessions, Monday to Friday for 5.1 weeks. IGRT with real-time image fusion with cone beam CT was used 3 times a week, in virtual simulation and at each treatment session, all patients were prepared as follows: strict empty rectum and comfortably full bladder. The planning objectives were as follows: prescription dose at planning target volume (PTV) V100 > 95%, restriction doses for rectum were V43.9 < 50% and V65.79 < 15% and for bladder V57.02 < 50% and V70.18 < 15%. In all plans, quality control was used with prior in vivo measurement with an arc check device. Maximum androgen blockade (MAB) was used at the discretion of the treating physician. The Phoenix consensus definition was used to determine biochemical failure, which is described as a prostate specific antigen (PSA) elevation of 2 ng/ml above the nadir achieved at follow-up9.
Acute toxicity was defined as gastrointestinal and genitourinary side effects following radiotherapy treatment occurring during treatment and up to 6 months after the end of treatment and was measured using the RTOG/EORTC10 scales, as was chronic toxicity, which was defined as gastrointestinal and genitourinary side effects after 6 months of treatment.
Results
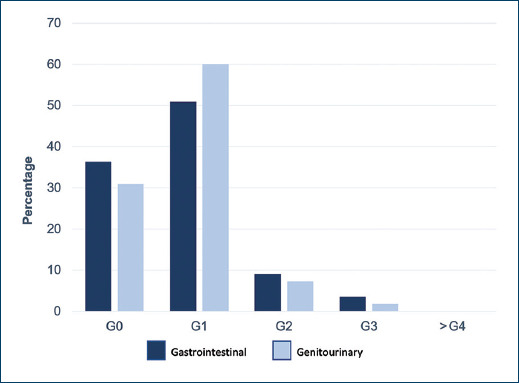
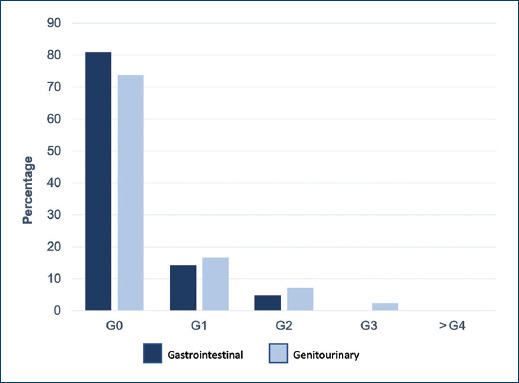
Fifty-five patients fulfilled the inclusion criteria. Demographic, clinical and pathological characteristics are presented in Table 1. Mean follow-up was 20.7 months. The mean age at baseline was 68.9 years (56-85 years), the mean antigen level at baseline was 34 ng/ml (5.5-231 ng/ml), 9.1% were classified as low-risk, 29.1% as intermediate risk and 61.8% as high-risk according to the D’Amico risk classification11. As for the patients who received MAB, 34.5% received it < 24 months, 52.8% received it for 24 months, 9.1% received it > 24 months and 3.6% did not receive it. 74.5% of patients received elective pelvic lymph node irradiation. The mean PTV coverage was V100 = 97.1% (94.4% - 99.87%). The mean nadir PSA was 0.15 ng/ml (0 - 1.25 ng/ml), representing 100% biochemical control. The mean organ at risk dose for the rectum was V43.9 = 28.77%, V65.79 = 16.42% and for the bladder V57.02 = 25% and V70.18 = 17.9%. Acute gastrointestinal toxicity grade 1 or less occurred in 87.3% of patients, grade 2 in 9.1% and grade 3 in 3.6%. Acute genitourinary toxicity grade 1 or less in 90.9% of patients, grade 2 in 7.3% and grade 3 in 1.8%, (Fig. 1). For chronic gastrointestinal toxicity, grade 1 or lower 95.3%, grade 2: 4.8%, there were no cases of grade 3 or higher; for chronic genitourinary toxicity, grade 1 or lower 90.5%, grade 2: 7.1%, grade 3: 2.4%, (Fig. 2).
Table 1 Demographic, clinical and pathological characteristics
| n = 55 | % | |
|---|---|---|
| Age (years) | μ: 68.9 years | |
| 50-59 | 5 | 9.1 |
| 60-69 | 24 | 43.6 |
| 70-79 | 24 | 43.6 |
| 80-89 | 2 | 3.7 |
| TNM Classification | ||
| T1 | 17 | 30.9 |
| T2 | 29 | 52.7 |
| T3 | 9 | 16.4 |
| N0 | 52 | 94.5 |
| N1 | 3 | 5.5 |
| M | 0 | 0 |
| Gleason | ||
| Group 1 (≤ 6) | 13 | 23.6 |
| Group 2 (3 + 4) | 13 | 23.6 |
| Group 3 (4 + 3) | 5 | 9.1 |
| Group 4 (8) | 15 | 27.7 |
| Group 5 (9,10) | 9 | 16 |
| Initial PSA (ng/ml) | μ: 34 ng/ml | |
| < 10 | 13 | 23.6 |
| 10-20 | 16 | 29.1 |
| > 20 | 26 | 47.3 |
| Risk group | ||
| Low | 5 | 9.1 |
| Intermediate | 16 | 29.1 |
| High | 34 | 61.8 |
| MAB | μ: 20 months | |
| No blockage | 2 | 3.6 |
| < 24 months | 19 | 34.5 |
| 24 months | 29 | 52.8 |
| > 24 months | 5 | 9.1 |
| Elective lymph node irradiation | ||
| Yes | 41 | 74.5 |
| No | 14 | 25.5 |
Discussion
Currently, there are no studies on moderate hypofractionation in prostate cancer with VMAT that analyse biochemical control and toxicity in the Mexican population. Our study is the first report with advanced radiotherapy techniques that corroborate the results obtained in the international literature, presenting an excellent biochemical control and an adequate toxicity profile. This could be explained by the fact that most patients were candidates for maximum androgen blockade and that a longer follow-up period is still needed. However, our results are encouraging in terms of the technique with which we administer the treatments and their quality controls.
Three large randomised controlled phase III studies, CHHiP, PROFIT and RTOG 041512-14, have shown that there is no inferiority in the use of radiotherapy with moderate hypofractionation techniques over conventional fractionation in all oncological variables. Thus, this treatment should be the standard in centres that have the technology for its implementation. Our study found the same results in the Mexican population. The meta-analysis by Yin et al shows that biochemical control in even moderate hypofractionation is superior to conventional fractionation with an RR= 0.8, 95% CI: 0.68-0.95, p=0.009, which is in accordance with our results in biochemical control. The data for chronic gastrointestinal toxicity reported by Pollack et al were: grade 0: 28.2%, grade 1: 53.7%, grade 2: 16.1%; grade 3 or higher: 2%; and for genitourinary toxicity: grade 0: 3.4%, grade 1: 51.7%, grade 2: 40.9% and grade 3 or higher: 4%. These results are similar to those found in our study, with the exception of grades 0 and 1, which may reflect under-reporting of symptoms associated with sexual function. Due to the long natural history of prostate cancer, our study still needs to follow up patients more closely to verify that the biochemical monitoring data remains as high as reported and that the trend in chronic toxicity is the same as before.
In addition, our hypofractionation system has allowed us to treat more patients in less time by freeing up accelerator slots without compromising safety and efficacy.
Conclusions
Moderate hypofractionation in prostate cancer in the Mexican population of the HRAE "Centenario de la Revolución Mexicana" has excellent biochemical control and an adequate toxicity profile. This should be the standard treatment in centres with the technology for its implementation.
Our protocol reduces treatment time from 8 to 5.1 weeks, is more comfortable for patients and frees up slots on the linear accelerator, allowing our institution to treat more patients in less time, without compromising biochemical control and without increasing toxicity.











 nueva página del texto (beta)
nueva página del texto (beta)