Introduction
The most common form of the right ventricular outflow tract obstruction (RVOTO) is pulmonary valve stenosis. Pulmonary valve stenosis (PE) occurs remotely in 8-10% of congenital cardiopathies but is often associated with other congenital lesions. Valvular, subvalvular and supravalvular stenosis tend to be congenital and can be associated with genetic syndromes including Noonan, Alagilole and Williams syndrome as well as congenital rubella. Pulmonary stenosis can also be an acquired infection, for example, in cases of rheumatic heart disease, carcinoid heart disease, lumps, infectious endocarditis, or secondary to traumatism1.
Case presentation
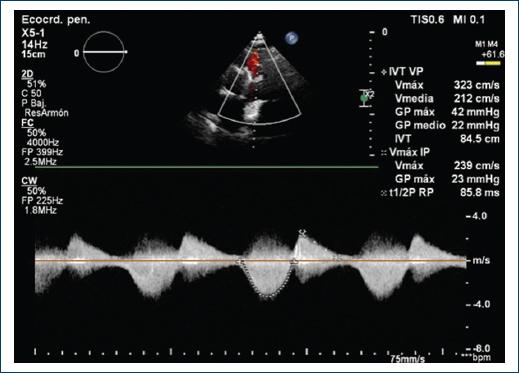
A 40-year-old female patient with a history of congenital complex heart disease: Ventricular septal defect (VSD) and infundibular stenosis both of them corrected with VSD closure and biological pulmonary valve bioprosthesis placement when she was 8 years old. The patient mentions a diseased sibling who died 7 days after being born due to a non specified congenital cardiopaty. Within her gynecologic history we find three pregnancies, one vaginal delivery, one cesarean section, and one current pregnancy of 25 gestation weeks. Her condition began within the previous 5 months with fatigue and dyspnea associated with lesser than usual activities, which is why she attended a prenatal care appointment from where she was referred to the cardiology service. During the physical cardiovascular exploration, she presented with rhythmical arterial pulses of adequate intensity and amplitude. Thorax malformations nonexistent, apex in the fifth intercostal space, anterior axilary line, 2.5 cm area with low left paraesternal lift. During cardiac auscultation: S1 normal, S2 split at the expense of P2, no S3 or S4. Holosystolic murmur in tricuspid area, intensity III/VI, regurgitant, augmented with Rivero Carvallo’s maneuver. Pulmonic area with mesotelesystolic murmur, intensity IV/VI, ejective, increasing-decreasing, as well as an early diastolic murmur. An EKG was requested, it showed complete. An EKG was requested, it showed complete right bundle branch block blockage (RBBB) and data of hypertrophy in the right ventricle (Fig. 1). A transthoracic EKG was taken which reported a left ventricle with a FEV1 of 53%, systolic flattening of the interventricular septum due to the overload of the right ventricle, Grade III diastolic dysfunction, dilated and hypertrophic right ventricle with preserved systolic function. No data of residual VSD. Right atrium severely dilated. Moderate tricuspid insufficiency, pulmonary artery systolic pressure (PASP) of 7 mmHg with low ecocardiographical probability of pulmonary hypertension. In the pulmonary valve an endoprosthesis with Vmax 5.5m/s was observed, peak gradient 124 mm Hg, mid gradient 68 mm Hg, valvular area by 3D planimetry of 1 cm2 compatible with severe pulmonary stenosis, in addition with Color Doppler jet area pulmonary insufficiency can be observed, classified as moderate because of a vena contracta of 4mm and a vena contracta’s area of 0.9 cm2 (Fig. 2). Due to findings compatible with bioprothesic pulmonary valve stenosis and because of her current pregnancy, she was scheduled for a plasty with baloon of said valve.
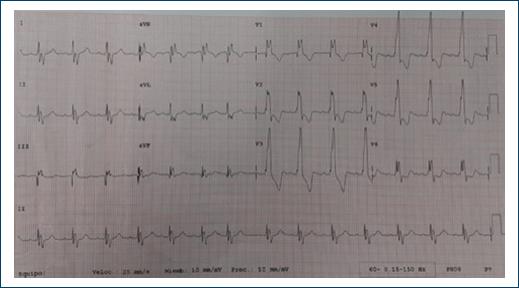
Figure 1 Electrocardiogram, 12 derivations, synus rythm, cardiac frecuency of 100 bpm. P 80 ms, PR 160 ms, rSR in V1-V6 to QR +120°, QRS 120 ms, no ischemia, lesion or necrosis.
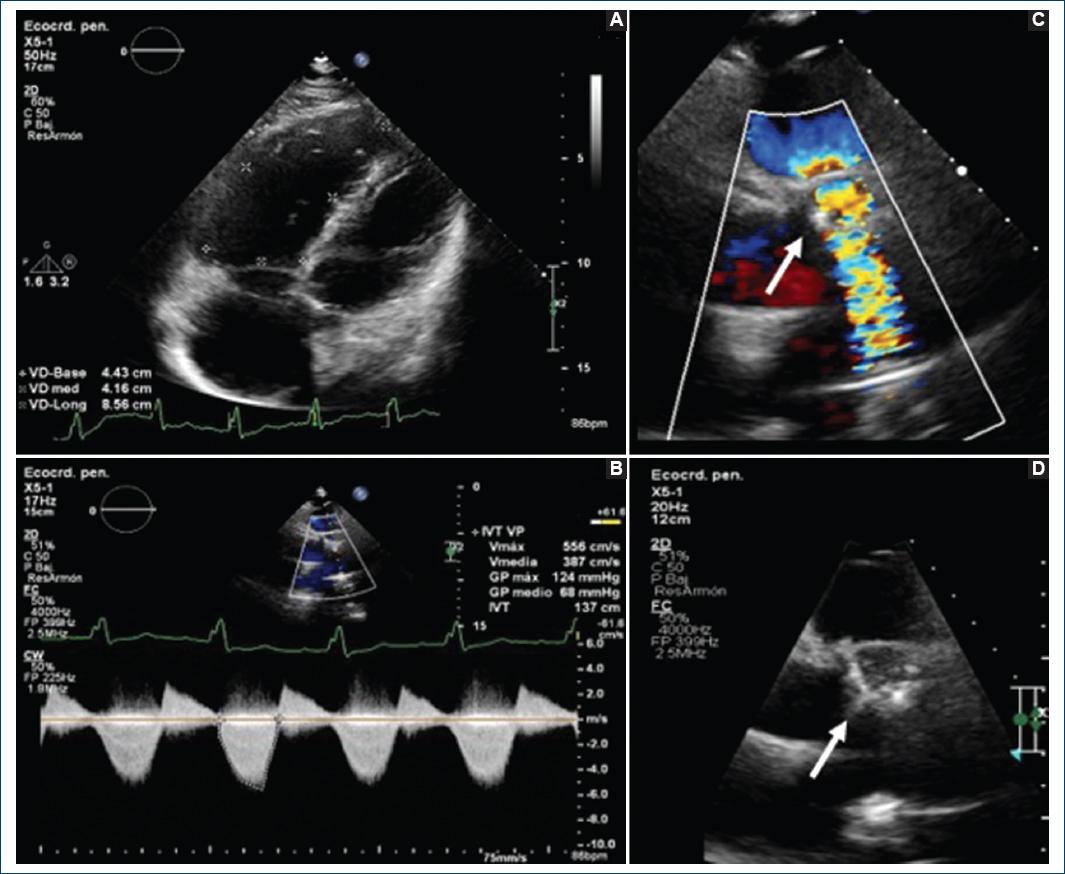
Figure 2 Transthoracic echocardiogram A: apical proyection 4 cameras. A dilated and hypertrofic right ventricle is observed B: continues Doppler. V max 5.5, G max 124 mm Hg, G mid 68 mm Hg. C and D: short axis projection at great vessels level. Here can be observed a bioprosthetic pulmonary thickened valve with turbulent flow by Doppler color (arrow).
Said procedure was done un the hemodynamic ward with the presence of the clinical and interventional cardiology services along with echocardiography, vascular surgery, cardiovascular anesthesiology, obstetrics and gynecology and the maternal-fetal medicine department of our hospital. With previous placement of a protection lead apron over the patient’s abdomen, a right femoral approach was made with modified Seldinger thecnic, a right catetherism was made with 5 Fr pigtail cathether and multipurpose 5 Fr MPB. Subsequently with a Super-Stiff 0.035 × 260 mm guide a femoral introducer was exchanged by a 12 Fr and a PTA MAXI LD 20 mm balloon was moved forward, positioning itself within the ring of the pulmonary prosthesis and dilating itself in three occasions until the disappearance of the balloon’s groove for the amplification of the infundibulum and the pulmonary bioprosthesis (Fig. 3).

Figure 3 Right catheterization. A-C: a pulmonary bioprosthesis can be observed and passage of the balloon catheter trough the ring of the pulmonary bioprosthesis. D-F: dilation with balloon within pulmonary bioprosthesis with disappearance of the balloon’s groove due to its total expansion.
A measurement of the pulmonary gradient was ade pre and post valvuloplasty (93 mm Hg and 26 mm Hg, respectively), showing a reduction grater tan 50%, finalizing the procedure successfully. A control transthoracic echocardiogram was made in the room, without any evidence os acute complications, corroborating the reduction of the pulmonary gradient with V. maxim 3.2 m/s, maxim gradient 42 mm Hg and mid gradient 22 mm Hg, continuing with moderate pulmonary insufficiency (Fig. 4). A fetal ultrasound was also made by the maternal-fetal medicine service without any evidence of complications for the binomy. The patient was released with an appointment at external consultation for monitoring and a cesarean section was programmed at 37 weeks of gestation without any complications. At present, the patient has satisfied parity and is scheduled for a mechanical pulmonary valve replacement, awaiting procedure.
Discussion
The pulmonary valve anomalies, the subvalvular area or supravalvular area can lead to the obstruction of the TSVD. PS can be valvular, subvalvular (infundibular) or supravalvular, being the valvular PS, the most common form conforming the 7-10% of congenital heart diseases. There is a slight predominance in the female gender and 2% of familiar cases do not have a genetic cause. The main consequence of PS is the overload of pressure of the right ventricle. Whose grade is dependent on the gravity of the stenosis. The overload of pressure of the right ventricle results in the increase of contractility and dilation, which leads to an increase in the tension of the wall and compensatory hypertrophy of the right ventricle2,3. The increase of the muscle mass allows the right ventricle to keep a normal cardiac output. The long-term volume load can cause diastolic dysfunction of the right ventricle with elevation of the end-diastolic pressure of said ventricle. The dilation of the right ventricle and the tricuspid ring form the substrate for the functional tricuspid regurgitation which aggravate the volume overcharge and is the cause of the symptomatology in patients. Said symptoms can be developed in patients with moderate to severe stenosis and mainly include dyspnea and fatigue with any effort like it happened with the patient of our case. In severe stenosis cases, the right ventricle cannot increase the cardiac output, which can cause thoracic pain by effort, syncope and sudden death. Right cardiac insufficiency is uncommon in adults but can happen if the severe disease remains untreated. For the diagnosis, the echocardiogram is the most common image technic and allows the evaluation of the location of the obstruction, the morphology of the valve and the degree of stenosis. Furthermore, information can be obtained about the TSVD, the pulmonary ring, the pulmonary arteries and the size and function of the right ventricle. The definition of severe PS according to the guidelines of the American Heart Association (AHA)/American College of Cardiology (ACC) from 2014 for the treatment of patients with valvular heart disease is a V. maxim over 4 m/s or a maximum gradient superior to 64 mm Hg. In this case, the patient had a V. maxim of 5.5 m/s and a maximum gradient of 124 mm Hg. In the guidelines of ACC/AHA of 2008 for the handling of adults with congenital heart disease, severe pulmonary stenosis is defined as a maximum gradient superior to 50 mm Hg, moderate when the gradient is from 30-50 mm Hg and minor when is inferior to 30 mmHg4. The balloon valvuloplasty can provide a certain degree of relief for the dysplastic pulmonary valves and is a reasonable first line option5. When the balloon valvuloplasty is not enough, a surgical intervention can be considered or a transcatheter pulmonary valve replacement if it is available. After the repair of complex cardiac defects, bioprosthetic pulmonary valves are often needed to maintain the function of the pulmonary valve. Many patients with bioprosthetic pulmonary valves require another surgery due to the inevitable failure of the valve6. The balloon valvuloplasty is a highly effective and safe method for the treatment of acute and chronic congenital pulmonary diseases. The balloon valvuloplasty of a bioprosthetic stenotic pulmonary valve can be an effective palliative procedure to delay the future valvular replacement. However, the balloon valvuloplasty of bioprosthetic valves in pulmonary positions has rarely been done. There have been some cases that reported successful dilations and better posterior clinics7,8. The American Heart Association and the American College of Cardiology recommended the following handling plans in their 2008 guidelines:
– Asymptomatic patients with a Doppler maximum gradient > 30 mm HG can be monitored every 5 years with electrocardiogram and Doppler echocardiography.
– Asymptomatic patients with a Doppler maximum gradient > 30 mm HG can be subjected to monitoring every 2-5 years with Doppler echocardiography.
– A valvulotomy with balloon is recommended for asymptomatic patients with a Doppler maximum gradient > 60 mm Hg.
– A valvulotomy with balloon is recommended in symptomatic patients with a Doppler maximum gradient > 50 mm Hg and a Domingo pulmonary valve.
– A surgical intervention is recommended for severe valvular stenosis with severe pulmonary insufficiency, hypoplastic pulmonary ring, subvalvular stenosis, or supravalvular stenosis9.
Due to the fact that the patient had subvalvular pulmonary stenosis as a kid, she required a replacement with bioprosthetic pulmonary valve. Unfortunately, the valvulotomy with balloon is not as effective on Domingo valves, which is why surgery is the preferred option10. The valvulotomy with balloon can be worth it if the Doppler maximum gradient is > 60 mm Hg in asymptomatic patients or if the Doppler maximum gradient is > 50 mm Hg in symptomatic patients. Surgery must be considered in patients that have undergone concurrent cardiac surgical procedures. The relevance of this reported case lies in the fact that it is about a patient with stenosis of a bioprosthetic pulmonary valve placed during her childhood. Said valves have an approximate life spam of 10 years, posterior to which it must be replaced due to the high risk of disfunction or valvular stenosis. However, the patient had already had that valve for over 30 years, which is why the failure was expected and a replacement with a mechanical valve had been indicated. Besides everything that was previously mentioned, the patient was pregnant, which increases the risk of complications. It is well established that pregnancy and delivery of a child lead to physiological changes that require the adjustment of the cardiovascular system. These changes are tolerated by pregnant women without heart disease but expose women with cardiovascular disease to great risks which is the reason why the prevention of cardiovascular complications must be the first objective of any cardiologist involved in the handling of pregnant patients with congenital of acquired heart disease. Slight or moderate PS are well tolerated during pregnancy with a good maternal-fetal prognosis11. Nevertheless, in patients with severe PS, pregnancy can lead to severe cardiac insufficiency or arrhythmias, which is why in this case and with the support of multiple departments of our hospital and despite the lack of evidence in the literature it was decided to perform in the General Hospital of Mexico “Dr. Eduardo Liceaga,” the first valvuloplasty with balloon of a bioprosthetic pulmonary valve on a pregnant patient, which had a successful result.











 nueva página del texto (beta)
nueva página del texto (beta)