Introduction
Technological advances have allowed radiation to be applied precisely to the desired site, managing to administer higher doses to the tumor, avoiding, or minimizing unnecessary radiation to nearby organs. It is understood that radiotherapy is a little known discipline, hence the importance of showing how it works and what is the process that is followed to achieve successful treatments since both malignant and benign tumors are treated and it is important that other medical specialties know this.
What is radiotherapy?
Radiotherapy is a treatment that consists of the emission of high-energy radiation that originates from a machine (linear accelerator), and goes to a certain area; it is considered that 60-70% of patients diagnosed with cancer will require treatment with radiotherapy at some point in the course of their disease1. It is also useful in benign pathologies such as: keloid scar, Graves’ ophthalmopathy, and arteriovenous malformations to name a few2.
Ionizing radiation is used to forms ions (electrically charged particles) and deposits energy in the cells of the tissues, this energy damages the genetic material (DNA) of the cells, causing them to lose their ability to divide and proliferate3. Radiation does not immediately destroy cancer cells, so it takes days or weeks of treatment for the DNA to be damaged enough for these cells to die4.
To give this treatment, a machine (linear accelerator) is used that generates high-energy photons (X-rays) that point to the site to be treated. A linear accelerator is a piece of equipment that uses microwave technology to accelerate electrons located in a waveguide. These electrons collide with a metal target to produce high-energy X-rays, which are shaped as they exit the machine to form a beam that resembles the shape of the patient’s tumor using a multileaf collimator. The beam exits from a part of the accelerator called a gantry, which can be rotated around the patient5.
The technology available is extraordinary and radiation oncology has evolved to become a highly sophisticated specialty due to the incorporation of computer tools, managing to obtain and transfer digital medical images, in addition to the development of planning systems in such a way that it evolved from a 2D radiotherapy based on X-ray to 3D radiotherapy based on volumetric images such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET-CT)6,7.
What is tomotherapy?
Tomotherapy is a charged particle linear accelerator that is specialized in rotational radiotherapy treatments using a fan-shaped radiation source and uses a table that moves at a constant speed during treatment. Its design reminds us more of a computerized axial tomography equipment, it has a donut-shaped gantry and inside is the compact linear accelerator that can rotate around the patient, and this helical linear accelerator generates an X-ray beam with energy of 6 megavolts (MV). The beam exits through primary collimators and generates a fan-shaped beam, the width of this beam is 1.0, 2.5, or 5.0 cm and laterally it reaches 40 cm. The fluence of this beam is modulated by a binary multileaf collimator, composed of 64 tungsten plates with a width of 6.25 mm each. Intensity modulation is achieved by varying the fraction of time that the different blades are open or closed. It has a mega voltage computed tomography (MVCT) detector mounted in front of the source that has the purpose of control and verification of treatments, in this way image-guided radiotherapy (IGRT) is performed in each treatment. The accelerator rotates at a constant speed while the table together with the patient moves longitudinally until completing the volume to be radiated8 (Fig. 1).
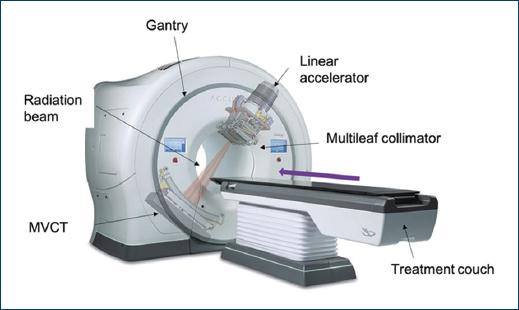
Figure 1 Basic diagram of a tomotherapy unit showing how the radiation beam exits through multileaf collimators that give it the shape of the tumor while the table moves longitudinally and the gantry rotates.
The advantages of tomotherapy over other linear accelerators are that the patient can be treated up to a length of 135 cm without repositioning and two or more volumes can be treated at the same time due to the characteristics of the equipment already mentioned, making the treatment faster and more comfortable for the patient, example: patients diagnosed with medulloblastoma, ependymoma grade II-III, leukemia, and embryonic tumors of the central nervous system (CNS) in whom the treatment field is required to include the skull and the entire bone marrow spinal. It has a high precision because all the treatments are guided by image, and verified in each fraction, this allows to carry out treatments with hypofractionation (higher dose in fewer fractions), for example: breast cancer9, also with this precision it allows the radiation dose to be reduced to the organs surrounding the tumor, thus reducing the toxicity that was experienced with other radiotherapy equipment (cobalt 60). It is a versatile piece of equipment, because we can perform intracranial and extracranial radiotherapy and radiosurgery techniques10.
Radiotherapy process
To carry out a treatment with radiotherapy, a set of procedures must be determined that are carried out from the assessment in the doctor’s appointment until the end of the treatment.
Medical office
As part of the multidisciplinary oncology team, an initial evaluation is carried out, where according to the diagnosis and staging of the disease, it is decided whether the patient is a candidate for this treatment, what is the sequence to follow (either neoadjuvant, concomitant or adjuvant) and the intention of treatment (curative or palliative).
Simulation
Administering multiple doses of radiation to exactly the same site requires immobilization of the patient, which must be easily reproducible throughout their treatment, this is achieved with the support of special fixation devices, such as thermoplastic masks (head and neck), breast ramp, and immobilizers (abdomen and extremities) (Fig. 2), these devices are placed on the simulator table that consists of a conventional CT scanner identical to those used for diagnosis, once the patient is positioned and immobilized, a volumetric acquisition and an image that is clear enough to define the area to be irradiated, then these images are sent to a special computer for planning.

Figure 2 Fixation devices used in simulations and treatments, depending on the site to be radiated, the device used to completely immobilize and be reproducible in each treatment.
At the end of the acquisition of the tomography, with the help of lateral and medical laser beams (arranged in the same way in the treatment bunker) marks or tattoos are made on the skin, with these marks we specify the isocenter and define our coordinate system that will be a reference in the linear accelerator that will allow to position and align the patient in all his treatments.
Planning
The computed tomography obtained in simulation is merged with MRI, PET CT, and/or contrast-enhanced CT to improve visualization of the organs.
With these images, the radiation oncologist defines the site to be radiated, in case of macroscopic disease, a Gross Total Volume (GTV) is outlined, a margin that includes subclinical disease, the possible routes of dissemination (based on the location and the histological type), lymphatic drainage and recurrence patterns, the sum of all this is known as CTV (Clinical Target Volume), and a margin that considers the variations due to the internal movement of the patient as well as the variations that it may have from a fraction to another, this is called the PTV (Planning Target Volume). The PTV is the volume at which radiation treatment is given11,12 (Fig. 3).
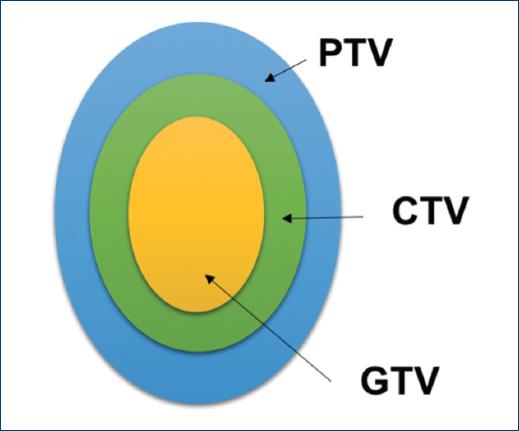
Figure 3 GTV: Gross Tumor Volume, defined as the tumor visible on images; CTV: Clinical Target Volume: Defined as GTV + subclinical disease (invisible invasion); PTV: Target Planning Volume: Defined as the CTV + margin that considers the variations between fractions.
In addition, the organs at risk are defined, that is, healthy organs that are close to the tumor (PTV) and whose function must be preserved as far as possible. The radiation oncologist also prescribes the total dose, fractionation, organs to be protected and their dose limits, later the medical physicist, who uses the simulated CT images to calculate the dose distribution, makes a treatment plan (according to physician specifications) that is optimized with a treatment planning system. The final treatment plan is presented to the doctor graphically and by means of a dose-volume histogram with the distribution of the dose both in the site to be irradiated and in the organs at risk. The radiation oncologist evaluates the treatment plan made by the medical physicist and verifies that it is appropriate for the treatment.
Quality control
Once the plan is approved by the radiation oncologist, the medical physicist verifies that the linear accelerator is capable of “mechanically” delivering the planned treatment, that it can reproduce head positions and movements of the multileaf collimator, position of the table, among others. And, in addition, verify that what is delivered “dosimetrically” is correct, that is, that the energy (dose) is that which comes out of the linear accelerator13.
Treatment
Once the quality control has been carried out, the procedure for starting treatment is as follows: the patient is positioned on the table of the tomotherapy team as simulated in the CT scan, using the tattoos made in the simulation as support, an image acquisition is performed with kilovoltage to what we know as mega voltage computed tomography (MVCT) to assess that it is correctly positioned when comparing the initial CT with the MVCT; with this information, the radiotherapy technicians make the necessary modifications to ensure the proper positioning of the patient. Once the radiation oncologist approves this verification, the treatment proceeds. These verifications are made to each patient before each of their treatment sessions.
Patient control
During treatment, the patient is evaluated in consultation by the radiation oncologist to find out if the treatment is being carried out according to what was estimated, to treat adverse effects if they occur, evaluate the result and once it is finished the patient is scheduled periodically to assess the chronic effects of radiotherapy.
All these steps are performed sequentially in each of the patients who are candidates to receive radiotherapy in the tomotherapy team. The time that this entire process takes is varied and depends on the workload of each institution. It generally takes 1-2 weeks, in the event of an oncological emergency (hemorrhage, spinal cord compression syndrome, etc.), this entire process is accelerated so that the patient receives treatment on the same day.
Tomotherapy in Mexico
According to the Accuray Latam & Worldwide database, 591 tomotherapy equipment (of this brand) are installed in the world; 18 are in Latin America, 13 are TomoTherapy model, 5 are RadiXact model. In Mexico there are 8 tomotherapy teams that are in: Centro Oncologico of Hospital Angeles de Chihuahua, Centro Universitario de Nuevo León, Centro de Cancer in Durango, Unidad de Especialidades Medicas in Zacatecas, Hospital Regional de Alta Especialidad del Bajio, Hospital Angeles del Pedregal, Fundacion de Cancer de Mama and Hospital Juarez de México14 (Fig. 4).
There are two ways to give radiation treatment with this equipment; Helical radiotherapy; in which the radiation is given in a continuous rotational way with a helical pattern, and Direct radiotherapy; where the gantry is fixed at a certain angle while the radiation is delivered, both forms of treatment are carried out while the table moves continuously longitudinally (Fig. 5).
Due to the new technologies and quality control that are available today, radiotherapy schemes have been modified, passing in some cases from conventional treatment to hypofractionation, that is, high doses of radiation are given in few fractions in selected patients and in this way the shifts in the team are optimized to be able to treat a greater number of patients, but the greatest advantage is for the patient because the overall treatment time is reduced and thus the costs of transportation, lodging, etc., are reduced.
The patients who are considered candidates for hypofractionation are mainly those with a diagnosis of breast cancer, both early clinical stages and locally advanced, and patients with rectal cancer (candidates for preoperative radiotherapy), since there are published studies that have shown that there is no difference in oncological outcomes (overall survival, disease-free survival, and locoregional recurrence) or toxicity15-17. It is important to mention that patients must be perfectly selected to carry out this type of treatment, since the success of the final result depends on it.
Conclusion
Tomotherapy is a helical linear accelerator that is installed throughout the world. Thanks to the ability of the team to perform image-guided radiotherapy, we have high precision in treatments, being able to take a technological leap that allows higher doses to be given to tumors and reducing the dose that reaches organs at risk, consequently, the patients have less morbidity and safe treatment is guaranteed even in benign pathologies. It is recommended to have trained personnel with well-defined activities to optimize the workflow.











 nueva página del texto (beta)
nueva página del texto (beta)