Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma, and it is responsible for approximately 30-50% of all new cases1,2. DLBCL represents a heterogeneous group of tumors with highly variable genetic abnormalities, clinical characteristics, responses to treatment, and prognosis3-5. The diagnosis of DLBCL is accomplished through histopathological studies and immunophenotyping. The following clinical criteria are currently used for determining the prognosis of this disease: clinical stage, functional state (ECOG), International Prognostic Index (IPI), LDH levels, and β2 microglobulin levels. Despite advances in immunotherapy (anti-CD20 therapy) as well as the incorporation of new cytotoxic agents (bendamustine), a select group of patients continues to have an unfavorable prognosis6.
The subdivision of DLBCL into two major biological categories based on their presumed cell of origin: germinal center B cell (GCB), and activated B cell (ABC)7.
Several molecular alterations have been identified in DLBCL, such as the expression of the NY-ESO-1 gene (New York esophageal squamous cell carcinoma-1), which is part of the group of cancer-testis antigen (CTA)8-9. This gene encodes a protein that is overexpressed in many cancers, but absent in normal tissue except for testicular10-11. This gene is found in a duplicated region of the X-chromosome and, therefore, has a neighboring gene of identical sequence. It has been used to diagnose and assess the prognosis of various types of cancer11-12, and its expression is restricted solely to immune-privileged germinal cells, which are the most immunogenic of this family13-14. It is abnormally expressed in a variety of cancers and is associated with the unfavorable evolution of cancer of the cervix and breast, as well as multiple myeloma and non-small cell lung cancer15-19.
Levels of expression of the NY-ESO-1 gene were analyzed in patients with DLBCL using quantitative RT-PCR (RT-qPCR) in real time and relationship between the clinical parameters and survival rate, the detection of NY-ESO-1 by RT-qPCR could be useful for disease prognosis and follow-up.
Materials and methods
Type of study
This was a prospective, descriptive observational, clinical study between April 2018 and June 2020.
Study population
This was a prospective clinical study with 112 patients with DLBCL who had previously provided signed informed consent forms. The histological diagnosis was established according to the World Health Organization (WHO) classification (SH, 2020). Approval for the present study was provided by the Ethics Committee of the Hospital General de Mexico “Dr. Eduardo Liceaga.” The informed written consents were collected from all enrolled patients and the entire study was performed based on the Declaration of Helsinki.
The study population was characterized according to their clinical parameters, including prior medical history, disease stage, and levels of lactate dehydrogenases (LDHs). The average age was 45 years (range 18-69), and 46.4% were male and 53.5% female. The patients were treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). Patients who showed a partial response to treatment were treated with dexamethasone, etoposide, and cisplatin as second-line chemotherapy at the discretion of the treating doctor. The survival global analysis was conducted after 5.3 years.
Lymph node biopsies
Lymph nodes from the patients were frozen in liquid nitrogen immediately after surgical excision and stored until RNA extraction.
Testicular tissue
Testicular tissue from a 60-year-old patient with prostate cancer was used to determine the levels of relative expression basal of the NY-ESO-1 gene.
RNA extraction and cDNA preparation
Total cellular RNA was extracted from the frozen tissue and the controls using TRIzol® Reagent (Life Technologies, Paisley, UK). The RNA was stored at –80°C until needed. A total of 2 μg of RNA was used for the synthesis of cDNA by means of the reverse transcriptase M-MLV (Life Technologies, Paisley, UK).
Quantitative real-time PCR assay
The mRNA expression levels of the NY-ESO-1 (Hs00265824_m1)19 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Hs00985689) genes were measured using the TaqMan® gene expression assay (Applied Biosystems, Foster City, CA, USA). The GAPDH gene was used as an endogenous control, and each sample was analyzed in triplicate.
The relative gene expression levels were calculated by the 2-ΔΔCt method. We used the median as cutoff between high and low expression.
Statistical analysis
The analyses between NY-ESO-1 gene expression and the clinical variables were performed using Chi-square test and Spearman test. The survival data were analyzed using the Kaplan–Meier method and compared with the log-rank test, considering p ≤ 0.05 to be statistically significant. The statistical program S.P.S.S. version 20 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, USA) was used for the analyses.
Results
Frequency of NY-ESO-1 expression at the mRNA level in DLBCL patients
The frequency of NY-ESO-1 gene expression was 46.4% (52/112). The levels of relative expression with respect to control (testicular tissue) were 0.2 times in Stages I/II, while in Stages III/IV, they were 1.5 times and 2.2 times, respectively, (Fig. 1). The expression levels were significantly different between Stages III and IV in comparison with Stage I/II, revealing a relation hip between the level of expression and advanced-stage disease (p = 0.007).
Association of NY-ESO-1 expression with clinical variables
The statistical analysis showed significant values for the parameters of LDH, clinical stage, and IPI (p ≤ 0.05). Elevated LDH levels in serum and a high IPI were associated with gene expression in 39.2% (p = 0.001) and 32.1% (p = 0.019) of the patients, respectively. In addition, 42.8% of the positives were associated with clinical Stage III or IV (p = 0.001) (Table 1).
Table 1 Associations between clinical characteristics and NY-ESO-1 expression in DLBCL patients
| Expression | No expression | |
|---|---|---|
| Median (range) | 58 (18-69) | 37 (19-65) |
| Sex | ||
| Male | (%) 20 (17.8) | (%) 32 (28.5) |
| Female | 32 (28.5) | 28 (25) |
| More than 1 extra nodal site | ||
| Yes | 32 (28.5) | 20 (17.85) |
| No | 20 (17.8) | 40 (35.7) |
| ECOG performance status greater | ||
| 0 | 0 | 4 (3.5) |
| 1 | 28 (25) | 48 (42.8) |
| 2 | 24 (21.4) | 8 (7) |
| 3 | 0 | 0 |
| 4 | 0 | 0 |
| Serum LDH level > normal | ||
| Yes | 44 (39.2) | 12 (10.7) |
| No | 8 (7) | 48 (42.8) |
| 0.001* | ||
| IPI | ||
| Low | 4 (3.5) | 28 (25) |
| Low-intermediate | 12 (10.7) | 20 (17.8) |
| High | 36 (32.1) | 12 (10.7) |
| 0.019* | ||
| Stage | ||
| I-II | 4 (3.5) | 40 (35.7) |
| III-IV | 48 (42.8) | 20 (17.8) |
| 0.001* |
*Chi-square value significance level p ≤ 0.05.
LDH: lactate dehydrogenase.
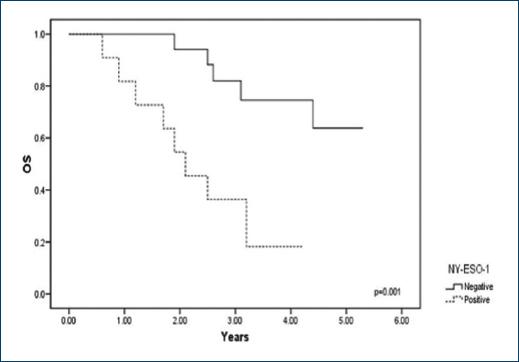
Expression of NY-ESO-1 and its relation to the survival rate
The study was performed over 5.3 years, and survival median at 3 years was 23.1% for the positive patients and 66.4% for the negative patients (Fig. 2). During this period, we observed that 76.9% (40/52) of the patients expressing NY-ESO-1 died. In contrast, 33.3% (20/60) of the negative patients died. In the statistical analysis, a log-rank value of p = 0.001 was calculated.
Discussion
Patients with DLBCL exhibit heterogeneous clinical characteristics, as well as variability in their responses to treatment and prognoses20-22. Although survival can be estimated based on clinical parameters (age, LDH levels in serum, extranodal site involvement, disease stage, and immunophenotype B), as well as molecular abnormalities (p53, BCL-2, BCL6, MUM.1, and Ki67), controversy exists regarding their utility as prognostic and survival markers23. As a result, it is of paramount importance to find new markers that could be incorporated to determine the prognosis of this disease.
We evaluated the clinic pathological relevance of NY-ESO-1 gene expression in patients with DLBCL at diagnosis who were admitted to the hematology service of the Hospital General de México. We decided to examine the expression of the NY-ESO-1 gene in patients with lymphoma, as it is a CTA present in various types of cancer and is associated with clinical factors such as poor prognosis and lower survival24-25. We confirmed that NY-ESO-1 gene expression is associated with the advanced stage of the disease, changes in the levels of LDH and the IPI, and survival rates. In DLBCL, there are no reports of an association between the expression of this gene and clinical parameters. Hudolin et al.26 analyzed the expression of the NY-ESO-1 gene in 24 samples of testicular tissue with DLBCL; expression was observed in 54.1% and was not correlated with clinical parameters or survival. We observed that the percentage of expression of the NY-ESO-1 gene in the stages of the disease in patients with DLBCL was 3.5% in Stages I-II and 42.8% in Stages III-IV. These results are consistent with the previous reports on melanoma in which a percentage of 3.34% was observed in Stage I and 9.52% in Stage II, and up to 45% in Stage III27. Similar results were reported in bladder and prostate cancer, where the frequency of expression increases with respect to the stage of the disease28-30.
Only two studies have measured the expression levels in metastatic esophageal squamous cell carcinoma and non-small-cell lung cancer using RT-qPCR, and elevated transcription levels were associated with advanced disease stages31-32.
The increase in the level of NY-ESO-1 gene transcription in patients with DLBCL is a finding of great importance; it could be a prognostic marker for this disease. In addition, the increase in advanced stages of the disease may explain its oncogenic role and the proliferative advantage it confers to tumor cells33-34.
Global survival is lower in patients who express NY-ESO-1, and these results concur with those reported for lung cancer, demonstrating that the expression of NY-ESO-1 is significantly associated with an adverse prognosis35. Similar data associating the expression of this gene with decreased disease-free survival have been reported for gastrointestinal and bladder cancer36. Other reports have examined the associations between the expression of the p53, bcl-2, and ki67 genes and global survival in patients with DLBCL and did not observe an association37. Rearrangements of the BCL-6 gene have been associated with 50% survival at 5 years in patients treated with R-CHOP, although the reported expression frequency was only 19%38. We have reported that the MAGE-A3 gene is associated with a decrease in survival in patients with DLBCL39.
NY-ESO-1 gene expression in patients with DLBCL may be helpful for identifying and stratifying risk groups, with other molecular marker of this disease that may benefit from new or intensified therapies.











 nueva página del texto (beta)
nueva página del texto (beta)