Introduction
Magnesium (Mg++) is a divalent cation with a molecular weight of 24.303u, the fourth most abundant in the body and the second at the intracellular level. It comprises approximately 0.03% of total body weight (99% intracellular and 1% extracellular), 95% is filtered in the glomerulus and 5% is excreted in feces1,2.
In the 1980s it was observed that physiological magnesium concentrations produce a reversible blockade of the N-methyl-D-aspartate (R-NMDA) receptor, playing a key role in cell activity, its main biochemical functions being: 1. - the synthesis and degradation of high-energy compounds by binding to adenosine triphosphate (ATP), forming a biologically active substrate [Mg++-ATP]; 2. - intervention in oxidative phosphorylation; 3.- the modulation of the transportation of ATP-dependent ions (sodium, potassium and calcium); 4. - it is a cofactor of more than 300 ATP-dependent enzyme systems; and 5. - in the cellular metabolism of carbohydrates, lipids and protein. The advancement of anesthesiology has seen enormous progress together with molecular biology, and magnesium sulfate (MgSO4) has been used in trans-anesthesia procedures as a sedative, analgesic and muscle relaxant, as well as an organ protector in different scenarios, for over 20 years1,3. We have conducted a literature review with the aim of describing the properties of magnesium, its mechanism of action and the use thereof in anesthesiology in recent years.
Methodology
We conducted a literature review with the use of keywords (anesthesia, magnesium sulfate, analgesia, muscle relaxation and organ protection) using the PubMed, Science Direct, Embase and Cochrane Library search engines. We focused on meta-analyses, review articles and controlled trials, however we focused on controlled trials in the case of uses with little evidence. The articles were evaluated by 3 people, who conducted a summary of each one and filed them in a folder; the information was written up at a later date.
Results
A total of 244 articles were obtained, 210 of which were discarded due to being repetitive, irrelevant, trials with faulty methodologies or not directly applicable to the anesthesia process, leaving a total of 34 articles.
Pharmacokinetics and pharmacodynamics
The term Magnesium Sulfate has been used in the field of medicine in a popular manner, however, it should be pointed out that the real chemical name is magnesium sulfate heptahydrate (MgSO4 7H2O). MgSO4 7H2O is available in 10-ml format and contains 1.5 g of MgSO4 and 150 mg of Mg++ (6 mmol or 12 mEq). Mg++ is absorbed by the small intestine, particularly when administered orally. When administered intravenously (IV), it has an immediate onset of action, with a peak effect in 10 minutes and a length of action of 30 minutes; the intramuscular (IM) route is slower and reaches a peak of action at 60 minutes with a half-life of 4 hours. Another route of administration is through nebulisation as an adjuvant in asthma crises and more recently at the epidural level1,4.
– Distribution: 99% is intracellular (53% to 60% is found in bone tissue, 20% to 27% in muscle tissue and 19% in soft tissue) and 1% is extracellular (60% ionised, 30% protein-bound and 10% complex anions). At the intracellular level, it is found mainly in ionised form in ATP, cytoskeleton, nucleotides or enzyme complexes, leaving a very small portion in non-ionised form. Muscle cells, especially cardiac, have a higher concentration of intracellular Mg++ (11-17mmol/L). The tissue depositions of magnesium sulfate are stable and a balance will be reached by day 41 to 181 in the event of replenishment1,4.
– Elimination: mainly renal (80%), 95% of which is reabsorbed (10-15% in the distal contoured tube (DCT) and the rest in the loop of Henle), with only 3-5% being eliminated in urine. It can increase or decrease depending on the plasma concentrations of Mg++ or certain medical conditions such as pre-eclampsia, where a decrease in clarity is observed before delivery compared to postpartum1,4.
With regard to pharmacodynamics, magnesium sulfate has multiple mechanisms of action, such as the activation of the sodium potassium ATPase pump, which can be inhibited in the presence of high concentrations of Mg++; competitive antagonism of calcium channels conferring the characteristic of blocking the release of presynaptic acetylcholine and increasing the threshold of postsynaptic action potential, blocking the release of catecholamines from the adrenal glands and adrenergic nerve terminals, as well as reducing the release of cytokines (IL-1, IL-6, TNF-a and substance P) and the inhibition of platelet aggregation; non-competitive antagonism of R-NMDA; sinusoidal and atrioventricular (AV) block, PR prolongation and QRS widening. Each of these is dose dependent, which involves a risk of toxicity at high doses1,2,4.
As with other drugs, anesthesiologists should be fully familiar with MgSO4 7H2O, as it needs to be administered with caution due to its pharmacological interactions. We should bear in mind that the anesthesiologist is in contact with all types of stable and critical patients (heart disease, pregnant women with pre-eclampsia or eclampsia, asthmatics, etc.), all of whose condition could worsen, exacerbate or who are undergoing an MgSO4 7H2O-based treatment on arrival; and all of them will need to continue to manage it or to start on it. MgSO4 7H2O features various pharmacological interactions that need to be taken into account by the anesthesiologist, including: 1.- interactions with other anesthetic drugs on reducing the consumption of hypnotics such as Sevofluorane and Propofol (its hypnotic properties could result in a reduction in the consumption of any hypnotic used in the trans-anesthetic, however there is no literature with regard to others), as well as demonstrating a synergistic effect with non-depolarising muscle relaxants (this is explained by a lower release of calcium); 2.- calcium is an antagonist of MgSO4 7H2O and is even used as a treatment in the event of poisoning; 3.- with antiarrhythmic drugs, a synergy with calcium channel blockers has been registered, which translates into a reduction in systemic blood pressure, likewise it reduces the serum levels of digoxin and increases the serum levels of quinidine; 4.- the use of diuretics such as thiazides promote the elimination of MgSO4 7H2O; 5.- some antibiotic drugs are capable of increasing its clearance; 6.- some corticosteroids are capable of reducing the serum levels of Mg++ (Table 1)1.
Table 1 Drug interactions
| Anesthetic drugs | Adjuvant: reduces the consumption of sevofluorane, propofol defluorane, opioids and non-depolarising neuromuscular relaxants |
| Calcium | Magnesium antagonist |
| Antiarrhythmic drugs | Synergy with Ca+channel blockers (responsible for
the antihypertensive effect) Reduces serum digoxin levels Increases serum quinidine levels |
| Diuretics | Thiazides increase the elimination thereof |
| Antibiotics | Aminoglycosides and amphotericin B increase the clearance thereof |
| Corticoids | Prednisone reduces serum Mg++ levels |
Ca+: Calcium; Mg++: Magnesium.
NMDA receptor
In light of the various mechanisms of Mg++ in our body and its participation in R-NMDA, this receptor, an ionotropic-type glutamate receptor, takes on significant importance. They have a heterotetrameric structure made up of three different subunits named GluN1-3, each of which has 4 transmembrane domains (M1-M4). The GluN1 subunit has eight isoforms, while GluN2 has four and GluN3 two. Functional NMDA receptors contain two binding subunits of GluN1 in combination with two subunits of GluN2 and/or GluN3 (Fig. 1)5.
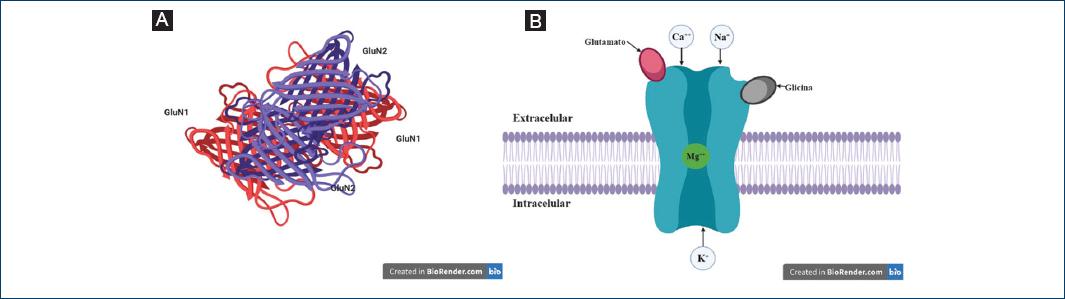
Figure 1 A: sub-units of the NMDA receptor. B: NMDA receptor; Glutamate, Glycine, extracellular, intracellular.
The simultaneous presence of two different ligands is required to activate it. The S1 subunit provides the receptor with stability, while the S2 subunit enables it to bind to the ligand, the gate opens through the rotation and lateral movement of M3, allowing for the entry of Ca++. The R-NMDA GluN1/GluN2 (the most abundant receptor) require two glycine molecules and two glutamate molecules, while the GluN1/GluN3 require glycine only in order to become activatived5.
Partial R-NMDA agonists and coagonists include: L-glutamate, D-glutamate, N-methyl-L-aspartate, D-aspartate and L-aspartate. Glutamate concentration determines the specific pattern of R-NMDA activation. Synaptically released glutamate reaches a cleft concentration peak of 1 mm and is then rapidly eliminated through diffusion and absorption, with a time constant of 1 ms. Once the coagonists and agonists have bound, the ion channel opens selectively for cations (especially Na+, K+ and Ca++), however, it is 10 times more permeable to Ca++ compared to Na+. Calcium is a key intracellular signaling molecule involved in many kinds of neural plasticity, but it is also a key mediator of excitotoxic cell death when present in excess;.
Finally, the antagonists of this receptor are entrusted with preventing the entry of Ca++ to the cell; some of the antagonists used in anesthesiology for the treatment of pain are ketamine and magnesium. Magnesium has a high affinity to GluN1/GluN2A and GluN1/GluN2B receptors, and intermediate affinity to GluN1/GluN2C and GluN1/GluN2D receptors6. The initial justification for the use of this drug as an analgesic is that a Gabaergic polarisation occurs during the pain process; in other words, a greater amount of gamma-aminobutyric acid (GABA) is released, resulting in the the activation of its receptor (R-GABA) and reducing the release of glutamate and, therefore, its activation, thereby promoting a pro-inflammatory response. This is now being used as a basis for the treatment of both acute and chronic pain, demonstrating its sedative effect, as well as its various pharmacological actions (Table 2).
Table 2 Pharmacological effects of magnesium sulfate and uses in anesthesiology
| System | Pharmacological effect | Use in anesthesiology |
|---|---|---|
| Nervous system | Sedation Analgesia (cerebral and neuraxial) Cerebral vasoconstriction Sympatholysis |
Hypnotic adjuvant (reduces PONV) Analgesic Neuroprotective in neurological surgery and in patients with pre-eclampsia (prevents seizures) Control of blood pressure |
| Muscles – Cardiac – Smooth – Skeletal |
– AntarrhythmicCoronary vasodilation
–Pulmonary: bronchodilationVascular: arterial vasodilationUterine: tocolysis (should no longer be used for this purpose) – Relaxation |
Heart rate control Cardioprotective Bronchospasm (not first choice) Control of blood pressure Not applicable. Adjuvant for non-depolarising muscle relaxants |
| Metabolism | Membrane stabilisation Reduces oxidative damage by regulating the Na-K-ATPase pump. |
Participates in analgesia and organ protection
Participates in analgesia, organ protection and regulation of the inflammatory response |
| Immune system | Modulation of the immune response
–Hypermagnisemia: inhibits cytokine release, risk of thrombosis. –Hypomagnisemia: release of free radicals, DNA mutations, risk of bleeding. Platelet anti-aggregant at high doses |
Modulates the inflammatory response
Possible increase in cardiovascular risk Possible increase in surgical stress, inflammatory response, and risk of intra-operative bleeding Possible reduction in thrombotic effects |
PONV: post-operative nausea and vomiting; ATP: adenosine triphosphate; DNA: deoxyribonucleic acid.
The nervous system
Sedative and anticonvulsant
Mg++ produces an inhibitory effect on the neuron by blocking R-NMDA, which inhibits the entry of Ca++ and prevents the release of other neurotransmitters, as well as activating neuronal apoptosis, hence it can be used as a sedative, anticonvulsant and neuroprotective. It has been shown to be effective in the prevention of seizures in patients with preeclampsia and pheochromocytoma, but not in other cases. Serum concentrations of between 2 and 3.5 mmol/L are generally regarded as therapeutic in pre-eclamptic patients. It is used to generate hypnosis and analgesia in anesthesia as it reduces the requirements for sevoflurane, defluorane, propofol and opioids. It is widely used in several different fields of medicine (neurology, pediatrics, gynecology, neuro-anesthesiology) due to its neuroprotective effect1,4,7-10.
Analgesia
Mg++ can modulate the transmission of nociceptive stimuli and the perception of pain by blocking R-NMDA and Ca++ channels in the central nervous system, regulating the release of neurotransmitters in the brain and spinal cord. More specifically, Mg++ has a depressant effect on the release of catecholamine in adrenergic nerve endings, the spinal cord and adrenergic sympathetic postganglionic fibers. Clinical trials have registered its use in bolus, and/or intravenous, intra-articular infusion, as an adjuvant in regional anesthesia, total intravenous, epidural and intrathecal anesthesia11,11.
The infusion of a low dose of 10-15 mg/kg/hour leads to an improvement in postoperative pain scores from 6 hours to 3 days post-surgery, thereby reducing the requirement for opioids. Intravenous MgSO4 7H2O can speed up the onset and prolong the duration of sensory block, motor block, relieve post-operative pain, without any additional side effects and without speeding up the onset of motor block in preeclamptic women undergoing spinal anesthesia. Compared to drugs such as dexmedetomidine, Mg++ does not cause reflex tachycardia or secondary hypotension when used as a hypotensive agent in the intra-operative period; does not produce reflex hypertension, and does not reduce cardiac output. While dexmedetomidine is associated more with bradycardia, although it is the ideal drug for controlled hypotension and provides better visibility in laparoscopic surgery. Several studies support the analgesic effect of Mg++ in orthopedic, cardiac, abdominal and otorhinolaryngological surgery, with a reduction in the requirement fo opioids, mainly alfentanil, fentanyl, morphine and remifentanil. It has been used as a drug in controlled hypotension. It has also been used during neuraxial block, where “it prolongs the duration of motor block and sensory block, increases the onset interval of motor block and sensory block when administered via the subarachnoid route and reduces it when administered via the epidural route”7,12–18.
MgSO4 7H2O is not classified as an antiemetic, however, it should be regarded as a prophylactic for postoperative nausea and tremors, as this characteristic is acquired by reducing the consumption of opioids. It should be pointed out that no mechanism of action directly related to the regulation of the nauseous centre has been discovered to date1,4,14.
Muscle relaxant
The reduction in intracellular Ca++ delays vesicular transport, in such a way that the presynaptic release of acetylcholine is lower, thereby increasing the postsynaptic depolarisation threshold, which translates into muscle relaxation. When used in anesthesia, this characteristic reduces the onset time, intubation doses, the requirement for non-depolarising muscle relaxants, and increases the duration of the subrachnoid block1,4,18.
MgSO4 7H2O functions as a negative inotropic in the cardiac musculature, prolonging the conduction time associated with its calcium-antagonist properties, preventing it from binding to myocyte troponin C. Its antiarrhythmic effect can be explained by sinus node (SN) depression by prolonging conduction and the AV refractoriness time, without altering the ventricular function. Magnesium bolus therapy after cardiac surgery has been shown to lead to a significant but brief increase in serum Ca++. In smooth muscle, Ca++ inhibition leads to mainly arteriolar vasodilation, although it has also been associated with a reduction in angiotensin converting enzyme (ACE). The use of MgSO4 7H2O in the intraoperative phase of abdominal, orthopedic and urological surgery in patients diagnosed with grade 1 and 2 arterial hypertension registered greater hemodynamic stability after 60 minutes of anesthesia1,2,16.
MgSO4 7H2O is also beneficial to the muscles when used a bronchodilator in refractory asthma. It is important to remember that it shouldn´t be used as the only treatment in such patients. It has been shown that the combination of nebulised MgSO4 7H2O + salbutamol can effectively improve forced expiratory volume in 1 second (FEV1) in children with bronchial hyperresponsiveness compared to the exclusive use of nebulised MgSO4 7H2O at 10 minutes and 20 minutes. MgSO4 7H2O, due to its relaxing, spasmolytic and anti-inflammatory properties, reduces coughing, bronchial hyperresponsiveness and bronchospasm during extubation. Indeed, it has been shown that patients receiving topical MgSO4 7H2O prior to intubation undergo less post-operative throat pain and swelling, as well as less hoarseness; this effect is even better than that registered with the use of lidocaine or ketamine and similar to the use of corticosteroids1,19,20.
In times gone by, MgSO4 7H2O was used as a tocolytic treatment in premature labour; however we now know it is of no use in such cases. Nevertheless, it should be taken into account that the recommended serum concentration to achieve the desired effect in pre-eclamptic patients is 4.8 mg/dL and that its use as a tocolytic has now been ruled out, as it has proven to be ineffective in these cases1,19,20.
Organ protection
Mg++ is entrusted with maintaining adequate intracellular ATP levels; inhibits intracellular influx and accumulation of Ca++ (blocking the “R-NMDA” calcium channels). This prevents the release of pro-inflammatory cytokines and the formation of free radicals, thereby reducing edema and oxidative cell damage. With regard to the neuroprotective activity of Mg++ in patients with hemorrhagic CVD undergoing neurosurgery, despite the fact that several studies have registered adequate efficacy, a meta-analysis conducted in 2015 demonstrated a lack of evidence to support the use of MgSO4 7H2O as a neuroprotective, however, it is still the main treatment for the prevention of seizures in patients with pre-eclampsia in the field of obstetrics. At the cardiac level, it has been described as an efficient drug for the prevention of arrhythmias in both cardiac and non-cardiac surgery1,23,24.
MgSO4 7H2O has also been used to limit the hypertensive response during intubation by controlling the hypertensive crisis during pheochromocytoma surgery; patients diagnosed with arterial hypertension as it decreases the mean arterial pressure (MAP) under general anesthesia, reducing the heart rate (HR) and, therefore, bleeding12-15.
Coagulation
The importance of Ca++ in coagulation has been widely documented. Mg++, by acting on R-NMDA, is involved in free Ca++ levels and therefore tends to alter the state of coagulation. It has been shown that patients with type-2 diabetes mellitus are more prone to coagulopathies with a reduced partial thromboplastin time, lower serum Mg++ levels, an increased prothrombin time and high ionised calcium levels. Moreover, Mg++ intake has been associated with being a preventive factor in thrombotic events, whereby the risk of stroke is reduced by 2% for each 100 mg/day increase in magnesium. Furthermore, the intra-operative administration of intravenous magnesium sulfate reduces blood hypercoagulability in patients undergoing laparoscopic surgery for colorectal cancer. Aravidan et al 2016 illustrated a reduction in bleeding and a better surgical field in patients undergoing functional endoscopic sinus surgery under total intravenous anesthesia upon the administration of MgSO4 7H2O. The trials published to date on the use of MgSO4 7H2O and its role in the prevention of coagulopathy in the trans-anesthetic period have yielded positive results, however, we still have insufficient evidence26–29.
Immune system
The participation of Mg++ in the immune system is impressive; a large amount of this mineral is capable of blocking Ca++ channels and reducing their concentration, thereby delaying the vesicular migration of various molecules such as interleukins, and reducing the inflammatory response. Moreover, Mg++ ions are enzymatic cofactors involved in deoxyribonucleic acid (DNA) repair mechanisms that ensure stability and genomic fidelity. Magnesium deficiency can also be associated with inflammation and increased levels of free radicals capable of causing oxidative damage to DNA and promoting the presence of cellular mutations, and a higher expression of magnesium transport channels has been observed in tumorous breast cancer cells, which increases the intracellular concentration of the mineral, thereby contributing to tumour growth through its function of increasing energy demand. This data, however, is scarce and inconsistent29,30.
This leads us to consider whether or not the use of MgSO4 7H2O is appropriate in cancer surgery. In this regard, it has been shown, as in other surgeries, to be effective in reducing opioid consumption, as well as in post-operative algological control, nausea and vomiting, waking up with no anxiety, and less bleeding, all of which with no changes in serum Mg++ levels, in gastric and abdominal cancer surgery, pituitary adenoma, among others. In addition, MgSO4 7H2O is used in opioid-free anesthesia, where cancer patients are among the few indications. Nevertheless, up to now there is no evidence that it is more effective than ketamine, dexmedetomidine or even lidocaine pursuant to the evidence on the use thereof in this patient group31–33.
Hypo and hypermagnesemia
Serum Mg++ levels are measured in the pre-anesthetic examination, paying special attention to patients with pathologies associated with hypo or hypermagnesemia, as irregular levels could be associated with trans-anesthetic complications and even death. It is of paramount importance that the anesthesiologist is familiar with the normal levels of serum Mg++ and is capable of recognising the symptoms associated with variations in these levels (Table 3).
Table 3 Levels of plasma Mg++ and symptoms
| Plasma levels | Symptoms | |
|---|---|---|
| Severe hypomagnesemia | ≤ 1 mg/dL or 0.5 mmol/L | Seizures, tetany, arrhythmias (ventricular extrasystoles, ventricular tachycardia, ventricular fibrillation, predisposition to digitalis toxicity), hypocalcemia. |
| Mild hypomagnesemia | ≤ 1.6 mg/dL or 0.8 mmol/L | Parasia, shaking, paresthesias, positive Chevosteck and trousseau signs, carpal pedal spasms, nystagmus, hyperreflexia, QT interval prolongation. |
| Normal | 1.7 to 2.2 mg/dL or 0.85 to 1.10 mmol/L | Asymptomatic |
| Mild | 3-4 mg/dL or 1.5-2 mmol/L | Nausea, vomiting, dizziness, headache, lethargy, hyporeflexia, hypotension and first-degree AV block |
| Serious | 10-12 mg/dL or 5-6 mmol/L | Flaccid paralysis, coma, respiratory depression, complete AV block and/or asystole |
Mg++: magnesium; mg: milligram; dL: deciliter; L: liter; AV: atrioventricular.
Hypermagnesemia can occur in chronic infectious diseases, diabetic ketoacidosis, Addison's disease, atherosclerosis, and in chronic kidney failure. Hypermagnesemia can also be iatrogenic, although such cases are usually very rare. The use of MgSO4 7H2O infusions has shown an increase in Mg++ of up to 3 mg/dL with no symptoms, while infusions used in obstetrics register a plasma concentration close to the therapeutic values for preeclamptic patients (1.7 mmo/L)9,34.
Hypomagnesemia is often associated with prolonged diarrhea, prolonged diuretic therapy, malabsorption syndrome, hyperaldosteronism and alcoholism2. Mg++ deficiency prevents the ATPase and free Ca++ pump from functioning, causing stiffness, causing muscle relaxation. Although it is theoretically correct to say that the requirement for non-depolarising neuromuscular relaxants may be higher during the trans-anesthetic period, there are no studies in this regard. Plasma concentrations below 0.7 mmol/L are regarded as Hypomagnesemia, while levels below 0.5 mmol/L are regarded as a serious condition. It has been documented that post-operative patients admitted to the ICU may suffer from hypomagnesemia, which involves a higher mortality rate (41% x 13% in patients with normal levels). Initial management should be geared to respiratory and hemodynamic support (if necessary) and the administration of magnesium should be started with 60-100 mg/kg of calcium gluconate or 20-30 mg/kg of calcium chloride, hydration, 1-2 mg/kg of furosemide, in addition to hemodialysis1,4,34.
Discussion
There is extensive literature on the use of MgSO4 7H2O in anesthesiology where it is used as an adjuvant in sedation, analgesia, neuromuscular relaxation, motor relaxation in neuraxial anesthesia, a prophylactic for post-operative nausea and vomiting in practically all kinds of surgery, whereby it reduces the consumption of hypnotics and analgesics. It has the same results in cancer surgery, although the implications of the specific mutations of each tumour that could be promoted by Mg++ are still unknown. Unfortunately, the level of evidence has not yet been determined and this is largely due to the lack of studies in this regard.
The anesthesiologist is also entrusted with taking care of patients with pathologies that interfere with levels of Mg++ and which could undergo complications in the trans-anesthetic period; nevertheless, the use of MgSO4 7H2O in the field of medicine is widespread, which is why patients arriving with an infusion of this drug can be accepted, particularly in the case of pre-eclamptic patients, where it is used as a neuroprotective, with the requirement of therapeutic plasma concentrations, meaning the partial or permanent suspension thereof in the trans-anesthesia period is not recommended, and such treatment can be initiated if the patient isn´t receiving it. Despite the fact it interacts with non-depolarising relaxants, there is no association between residual relaxation and patients with an infusion of MgSO4 7H2O, although this data is still inconsistent and the area needs to be studied10.
Anesthesiologists are not only entrusted with caring for healthy patients and scheduled surgery, but their extensive work includes caring for patients with pathologies that may be associated with irregular levels of serum Mg++, patients that are undergoing an MgSO4 7H2O-based treatment upon arrival, and which needs to be continued or started. Finally, anesthesiologists have to deal with complications in the trans-anesthesia period, where MgSO4 7H2O can be used, such as bronchospasms (both in patients with a history of asthma history and apparently healthy patients), uncontrolled hypertension, excessive bleeding and cardiac arrhythmias.
The intravenous dose schemes are 25 to 50 mg/kg followed by an infusion of 6 to 25 mg/kg/hour (Table 4), with the exception of the Zuspan scheme, a model dating back to 1985 used in obstetrics for the management of pre-eclamptic patients, in which a bolus dose of 4 g over 30 minutes is standard, followed by an infusion of 1g/hour, which has been shown to be effective in preventing seizures, but has not reached the therapeutic doses specified. Experts describe this scheme as safe and effective, which is why they highlight the importance of basing treatment on this scheme and not on reaching the therapeutic levels, which reduces the risk of secondary toxicity data7,9.
Table 4 Reported doses of MgSO4 7H2O in various scenarios
| Indication | Dose mg/kg bolus | Infusion mg/kg/hour | ||
|---|---|---|---|---|
| Anesthesiology Reconsider use in cancer patients. |
Sedation | IV sedation Postoperative tremor Convulsions |
20-50 50 |
5-20 10-40 |
| Analgesia | IV analgesia TIVA Local (peritocillary) analgesia Epidural analgesia Intrathecal analgesia |
20-50 20-50 2-5 50 mg (total dose) 0.5-1 |
5-20 10-20 |
|
| Muscle relaxant | Tracheal intubation Laryngospasm IV Bronchospasm Bronchospasm (nebulisation) |
30 15 50-100 40-150 DU |
40-50 | |
| Antiadrenergic response | Cardiopulmonary bypass Long QT syndrome Neonatal pulmonary hypertension Pheochromocytoma |
25-50 30-50 200 30-50 |
5-20 20-150 5-20 |
|
| Organ protection | Neuroprotection Cardioplegia Perioperative hypomagnesemia Eclampsia |
250 40-80 25-50 Bolus 4-6 g |
5-20 1g/hour |
|
MgSO4 7H2O: magnesium sulfate heptahydrate; IV: intravenous; TIVA: total intravenous anesthesia; g: gram; mg: milligram; hr: hour.
Conclusions
The use of MgSO4 7H2O has positive pharmacokinetic and pharmacodynamic properties for use in anesthesiology, and more specifically it has shown various qualities in the management of surgery patients such as analgesia, sedation, hemodynamic stability, reduced PONV and a reduced consumption of opioids and hypnotics. However, the use of MgSO4 7H2O beyond this area is not limited and it can also be used to treat patient-specific pathologies.











 nueva página del texto (beta)
nueva página del texto (beta)


