Introduction
Osteosarcoma (OS) is a primary malignant tumor originating from primitive osteoid-producing primitive mesenchymal cells and disorganized immature bone.
Jaw osteosarcoma (JOS) is rare, occurring in about 6% of OS1,2. It appears two decades later than long bone OS, at an average age between 33 and 36 years3. The risk of developing metastases is between 20% and 25%, whereas long bone OS is between 44% and 49%2. The average survival rate is better at 77% at 5 years, when the tumor is localized and the resection is complete4.
According to the WHO classification of OS, it can be classified as high grade, intermediate, and low grade. Most JOS are high grade and include the conventional type, comprising osteoblastic, chondroblastic, and fibroblastic forms, and other rare varieties such as giant cell-rich, well-differentiated fibroblastic, epithelioid, and small cell5, depending on the amount of osteoid, cartilage, or collagen produced by the tumor cells.
The etiology of OS remains unknown. There is an absence of consistent molecular alterations and the karyotype is often complex6. The tumor suppressor genes p53 and Rb1 are frequently mutated and appear to be involved in the onset of the disease7. It has also been established that the bone environment plays an important role in the development, progression, and chemoresistance of OS8. The difference in the clinical and biological behavior of long bone OS and JOS is due to the different microenvironment between the two sites. The major alterations involve p53 and RB1 with 80%-90% and 10%-39%, respectively, and to a lesser extent, ATRX, Dbl2, RUNSX2, and PTEN9 genes are affected.
Alterations in p16 protein expression correlate with the pathogenesis and progression of OS9 and there is a correlation between p16 negativity and the risk of an unfavorable outcome of OS10.
Immune infiltrates form the key component of the complex local environment of OS11. This microenvironment produces everything necessary for the control of proliferation, drug resistance, and cell dissemination12. Macrophages are the major representative of the immune infiltrate and tumor-associated macrophages control local immunity, angiogenesis, and regulate tumor cell migration13. The immune profile of JOS has been explored by immunohistochemistry, finding low levels of CD4 and CD8, as well as low numbers of T-lymphocytes associated with antigen 4 and programmed cell death protein. However, no association was found between the immune profile and clinicopathological findings14.
In angiogenesis, Jawad et al.15 compared the vascular endothelial growth factor response in long bone OS and JOS, finding less expression in the latter, which could explain its low metastatic potential and poor response to neoadjuvant therapy.
JOS can be part of some hereditary syndromes such as hereditary retinoblastoma, Li-Fraumeni syndrome, Paget’s disease of bone, Rothmund-Thomson syndrome, and Werner syndrome or as sporadic JOS, by far the most frequent form16.
The maxilla and mandible are affected with equal frequency; the tumor originates in the posterior part of the body of the mandible and in the horizontal branch rather than in the ascending branch. Lesions of the maxilla are located in the alveolar ridge, floor of the maxillary sinus, and palate rather than in the zygoma and orbital ring17. The most common presenting symptoms are enlargement and pain. Other features include facial deformity, tooth loss, paresthesia, toothache, bleeding, and nasal obstruction. JOS most commonly affects males18.
Radiologically, JOS may present with sclerotic or radiolucent lesions; the classic “sunburst” appearance is due to periosteal bone production. The other classic image is due to infiltration of the tumor along the periodontal ligament, enlarging the periodontal space called “Garrington’s sign”17. The diagnosis of JOS is based on radiological findings and cell morphology data. As for immunohistochemistry, attempts have been made to separate the various histological subtypes. Thus, osteonectin and osteocalcin have been widely used. Osteocalcin is specific for osteoblasts, while osteonectin is positive in other cells such as fibroblasts, endothelial cells, chondrocytes, nerves, and giant cells. Other markers are S-100 protein, SOX9, Ki67, Bcl2, p53, caveolin-1, and CD99. However, due to the heterogeneity of tumors, a conclusive profiling of histological subtypes has not been achieved, and therefore, histological assessment is still considered the “gold standard”19,20.
The classification of OS is based on their location and histological presentation. According to their location, they are divided into central, paraosteal, and periosteal. Histologically speaking, the main feature is the presence of the fundamental cell which is the osteoblast and malignant osteoid in the stroma. In addition, seven cell types have been reported, namely, chondroblast-like cells, fibroblast, histiocytes, myofibroblasts, osteoclasts, and angioblasts. Depending on the cell present and the type of matrix, OS is divided into osteoblastic, chondroblastic, fibroblastic, telangiectatic, low-grade fibroblastic, giant cell-rich, epithelioid, and small cell types18.
The prognosis of OS is usually determined by the Enneking system21 which evaluates the histological grade (G), the extension of the primary tumor (T) as intra- or extra-compartmental, as well as metastases to regional lymph nodes or other organs (M).
Among the histological subtypes, the chondroblastic variety is the most resistant to treatment and therefore has an adverse prognosis. The fibroblastic type responds to treatment and has a better prognosis22. The main prognostic criteria in JOS are tumor size and complete resection23. Complete resection is mainly difficult in the maxilla, therefore, local recurrence rather than metastasis is most frequent, consequently positive surgical margins are associated with poor prognosis24.
JOS is relatively radioresistant. Hence, high doses are used. Chemotherapy improves survival in non-metastatic long spindle OS. However, neoadjuvant chemotherapy helps by improving local control and decreasing the incidence of pulmonary metastases18.
JOS is a rare tumor of the oral region and an understanding of the histological aspect is required, reducing diagnostic difficulties by separating these tumors from benign diseases of these bones.
Materials and methods
A search for JOS was carried out in the archives of the Surgical Pathology Unit of the Hospital General de México, during the period 2002-2019. The following was obtained from the cases found: histopathological reports, clinical data, slides, and paraffin slides for complementary sections if necessary. Two surgical pathologists reviewed all histological material with light microscopy. For histological grading, the Broder’s classification system25 was used, which is based on the degree of cellularity, pleomorphism, mitotic activity, evidence of invasion, and necrosis, resulting in four grades of malignancy.
For specific grading details in bone neoplasms, the Unni classification26 was used. It grades tumors as low-grade malignancy or G1 if they are classified as Broder’s Grades 1 and 2 and high grade or G2 if they are classified as 3 or 4. Finally, these data were organized into stages according to the Enneking classification21, which has prognostic purposes.
Results
Eleven cases of JOS were found, of which 6 (54.5%) came from surgical specimens, 3 (27.3%) from slides and paraffin blocks, and 2 cases (18.2%) from biopsies.
The specific clinical diagnosis of JOS was made in 3 cases (27.3%), tumor only was diagnosed in 3 cases (27.3%), and with 1 case (9.1%). Diagnoses of nasofibroma, giant cell granuloma, osteochondroma, carcinoma, and granulomatous sialoadenitis were also made.
The tumors were most frequently found in women, 6 cases (54.5%) versus 5 cases (45.5%) in men. The average age was 36 years, with limits ranging from 5 to 65 years, 54.6% of the cases were found between 20 and 40 years of age. The most frequent location was the maxilla with 54.5% and 45.5% in the mandible, in both bones mainly on the left side.
The size of the tumor could only be determined in six cases, the smallest being 2 cm, the largest 15 cm, and the average 8 cm. In terms of bone location, all 11 tumors were central, none were paraosteal. Macroscopically, JOS are tumors with poorly defined boundaries, yellowish-gray color, sandy surface, firm consistency with chondroid, hemorrhagic, or necrotic areas (Fig. 1).

Figure 1 Chondroblastic osteosarcoma. Tumor with a stroma of pleomorphic cells, which form osteoid and undergo chondromatous transformation (× 300).
Histologically speaking, the tumor is made up of malignant, spindle-shaped mesenchymal malignant cells that produce osteoid or immature bone that infiltrates pre-existing bone trabeculae. They are morphologically classified according to their extracellular matrix. Regarding the 11 cases, 36.4% corresponded to the chondroblastic variety (Fig. 2), 27.2% to the osteoblastic variety (Fig. 3), and 9.1% to the following four varieties: well-differentiated fibroblastic (Fig. 4), giant cell rich (Fig. 5), epithelioid type (Fig. 6), and small cell JOS.

Figure 2 Osteoblastic osteosarcoma shows sarcomatous, pleomorphic stroma with calcified and partially ossified osteoid formation, which is invaded by tumor osteoblasts (× 300).

Figure 3 Well-differentiated fibroblastic osteosarcoma; well-differentiated spindle cell sarcomatous stroma, which forms osteoid (× 200).
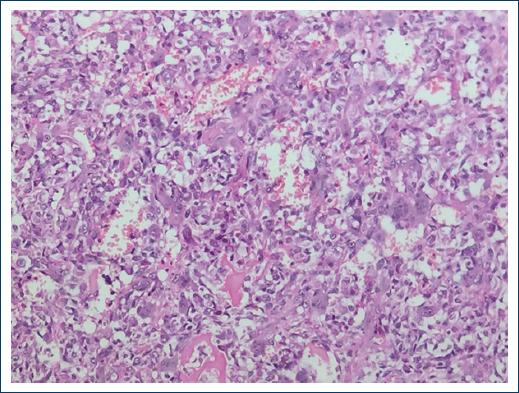
Figure 4 Osteosarcoma rich in giant cells. Sarcomatous stroma with osteoid formation surrounded by abundant multinucleated osteoclastic giant cells (× 200).
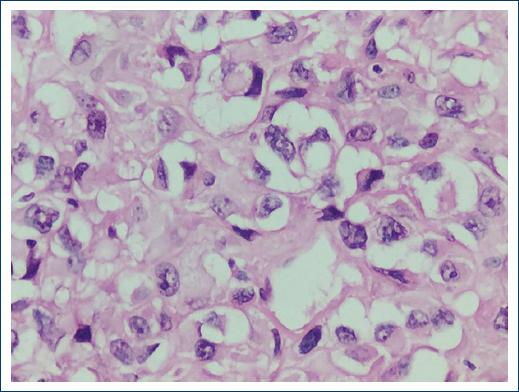
Figure 5 Epithelioid osteosarcoma. Sarcoma consisting of epithelioid osteoblasts which form osteoid in small numbers (× 400).
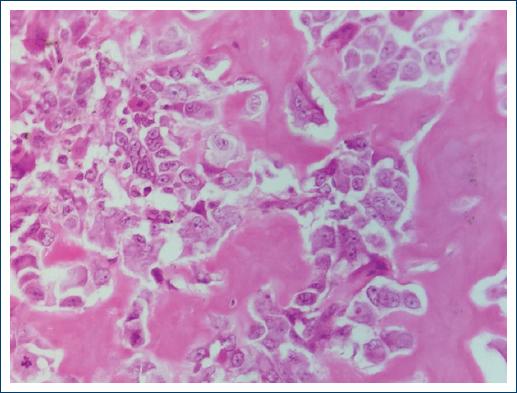
Figure 6 Small cell osteosarcoma. Tumor consisting of lymphoid-like cells, which form a small amount of osteoid material. (× 200).
For histological grade, the Broder’s classification25 was followed. Five cases (45.5%) were found to be Grade 2 or low grade (G1) in the Unni26 classification and 6 cases (54.5%) were found to be Grade 3 or high-grade malignancy (G2). Staging followed the Enneking system21, which comprises three stages, 9 of the 11 cases could be included as two cases corresponded to biopsies. As for nine cases, three were low-grade malignant (G1) and intracompartmental (T1), thus corresponding to Stage IA (G1,T1). Another case was also low grade (G1) but extracompartmental and corresponded to Stage IB (G1,T2). The remaining five cases were of high-grade malignancy (G2), two intracompartmental cases (T1), Stage IIA (G2,T1), and three extracompartmental cases (T2) Stage IIB (G2,T2) (Table 1).
Table 1 Classification by Enneckin System in 9 cases
| Stage | Number of cases | Grade | Number of cases | Compartment | Number of cases | Metastasis | Number of cases |
|---|---|---|---|---|---|---|---|
| I A | 3 | G1 | 3 | T1 | 3 | M0 | 3 |
| I B | 1 | G1 | 1 | T2 | 1 | M0 | 1 |
| II A | 2 | G2 | 2 | T1 | 2 | M0 | 2 |
| II B | 3 | G2 | 3 | T2 | 3 | M0 | 3 |
| Total | 9 | 9 | 9 | 9 |
*Two of the 11 cases were biopsies.
G1: Low grade. G2: High grade. T1: Intracompartmental. T2: Extracompartmental.
As for six of the 11 cases, it was possible to determine the condition of the surgical edges: 5 cases (83.3%) had tumor edges and 1 case (16.7%), with a history of radiotherapy, had no tumor edges.
Discussion
OSs are malignant bone tumors characterized by the formation of osteoid and immature bone from tumor mesenchymal cells27.
About 10% of OS occur in the head and neck, most of them in the mandible or maxilla28, with an average age between 33 and 36 years3. We found an average age of 36 years and Delgado et al.29 reported 28 years of age. With regard to sex, we found that the tumor was more frequent in women, which is consistent with the findings of Delgado et al.29 With regard to size, Delgado29 reported an average of 10 cm and we found 8 cm.
Kassir et al.30 pointed out that JOS occurs with equal frequency in the mandible and maxilla and has a similar prognosis, but we found that the maxillary bone is the most affected. Clinically speaking, the most frequent symptoms of JOS are enlargement and pain, which occur in 79% of cases, but establishing the correct diagnosis is not an easy task. Regarding the cases under study, the diagnosis of JOS was made clinically in only 27.3% of cases. In dental radiographs, two images are indicative of the diagnosis, one is the “sunray” and the other is the “Garrington sign”17. In our series, it was not possible to obtain the radiological signs. Metastases are rare, occurring in 5% of cases, and the lungs are the most affected31. JOS is divided into central and peripheral by location; our cases were central or intramedullary. In terms of behavior, they are divided into high-grade and low-grade malignant, 54.5% of our cases were high grade and 55.6% were intracompartmental. Histologically speaking, the conventional type of JOS is the most frequent with 36.4% of the chondroblastic variety27. In our series, in addition to the seven cases of conventional JOS, we found another four cases of rare JOS, such as well-differentiated fibrous JOS, which has a better prognosis than conventional JOS31,32, giant cell-rich JOS, and epithelioid JOS33,34 that have a similar prognosis to conventional JOS. In addition, we found one case of small cell JOS, which is more aggressive than conventional JOS35.
In terms of histological grade, 54.5% of the cases were within the high grade of malignancy, which is similar to that reported by Delgado29. In terms of staging according to the Enneking classification21, 55.5% were high-grade neoplasms and 44.4% had extracompartmental location (Table 1).
The prognosis of JOS depends mainly on two criteria: the size of the tumor and complete resection23, the latter being difficult to achieve due to the anatomical features of the region, mainly in the maxilla, which results in a poor prognosis24. In our cases, complete removal of the tumor was achieved in 16.7% of cases, lower than Delgado’s case report29, which was 43%, and the 5-year survival rate in the same author’s cases was 10%. Follow-up was not possible in our cases.
Death is secondary to local extension with vascular and neural infiltration31. Therefore, even though these tumors are rare, it is necessary to recognize them to detect them in time and be able to remove them completely to achieve prolonged survival or even cure.
This paper combines two important facts, the histomorphological varieties of JOS, the conventional variety and rare varieties, which have prognostic implications, as well as the staging according to Enneking’s criteria, which also have prognostic significance. We did not find these facts referred to in the literature.











 nueva página del texto (beta)
nueva página del texto (beta)


