Introduction
In the final months of 2019, a new type of coronavirus was associated to the development of pneumonia in the city of Wuhan, China1. Its appearance was traced back to the consumption of food (a water snake) at an open-air market in this city. A short time after this discovery was made, a beta-coronavirus, was isolated as the etiologic virus, causing what is now known as severe acute respiratory syndrome coronavirus 2 (SARS - COV 2). The evidence presented on the isolation of the virus included bronquio-alveolar lavage specimens, which were, in turn, submitted to indirect genomic sequencing by polymerase chain reaction (PCR) and viral culture. The dissemination of this new agent was quick to originate an epidemic in a matter of weeks. By February 2020, cases continued to increase, prompting the World Health Organization to declare it a pandemic in March 20201,2. Until now, there have been more than 10 million cases have been confirmed worldwide by the World Health Organization, including the first cases reported in China at the end of 2019. The infection has now spread through all continents except Antarctica. The accumulated incidence pertaining to each place where the pandemic propagates depends on certain factors, which include demographic density, the detection, report and application of tests, and strategies to reduce the spread of the virus1,2.
Transmission and clinical manifestations
The primary form of contagion of SARS-COV-2 is person-to-person contact. Contact with flügge droplets and exposure to the mucosa, whether it be nasal, oral, or ocular, to agents pertaining to the airway of infected persons who cough, sneeze, or talk are the most prevalent ways of contracting the virus. In addition, contact with surfaces where the virus is present and the afterward contact with the previously stated mucosa is a noteworthy form of transmission1,3. The virus can present itself in a symptomatic or asymptomatic fashion way, which varies depending on the severity of infection. The proportion of asymptomatic patients has yet to be further studied, as it is estimated that they comprise up to 30-40% of all cases reported. In addition, it is estimated that the incubation period for this virus varies from 1 to 14 days, with a median estimated time of 5 days to develop symptoms4.
The virus may be transmitted during the incubation period. The pathophysiology of this new disease is not well known, notwithstanding, some studies suggest that the virus may form a bond with the angiotensin 2 converting enzyme, a similarity shared with the already known SARS virus. An elevated viral load has been detected in the nasal mucosa and pharynx of patients as soon as symptoms develop. It is notable to say that asymptomatic patients may have a viral load as elevated as that of patients with severe symptoms5,6. Signs and symptoms associated with this disease are similar to those of a viral pneumonia, its gravity ranging from mild to severe. Approximately 80% of patients initiate with mild disease, 14% with severe disease, and 5% of patients are in need of critical care medicine. Some reports suggest that the severity of the disease is strongly correlated with age and preexisting comorbidities. Among the most common comorbities are cardiovascular disease, diabetes mellitus, hypertension, chronic pulmonary obstructive disease, obesity, and chronic kidney disease4,5.
The most common symptoms include fever, cough, dyspnea, myalgia, and fatigue. Among the less frequent are anorexia, sputum production, confusion, rhinorrhea, headache, anosmia, loss of taste, nausea, vomiting, diffuse abdominal pain, and diarrhea6. The vast majority of patients present one or more symptoms. We observed an average of 15% presented with fever, cough and dyspnea on onset of the disease4.
Laboratory anomalies in hospitalized patients with SARS-COV 2 pneumonia include leucopenia, with leukocytosis in some cases, lymphopenia, elevated liver enzymes, thrombocytopenia, anemia, hypoalbuminemia, or acute renal injury. More than half of patients are evaluated with < 90% of oxygen saturation. Some studies have related the severity of the disease, both in presentation and outcomes, to the elevation of biochemical markers, such as de elevation of lactic dehydrogenase (LDH), ferritin, interleukin 6 (IL6), and D dimer3,5,6. Some patients with severe disease have a laboratorial confirmation of an exuberant inflammatory response, comparable to a cytokine release syndrome or a cytokine storm, associated, in turn, with persistent fever and the elevation of the aforementioned biomarkers. As such, the elevation of these biomarkers has been associated with critical and even fatal disease5. The aggressive inflammatory response that occurs in this disease is intrinsically related to the damage caused in the respiratory system7. Thus, the severity of the disease is not something related only to the viral infection that is in intertwined with the host's response. Cytokine release in response to a sustained viral infection, specifically pro-inflammatory cytokines, may result in symptoms associated with sepsis. The attributing factor to approximately 28% of SARS-COV 2 deaths, as in these cases, uncontrolled inflammation leads to multiple organ failure, particularly renal, cardiovascular, and hepatic8.
Among known pro-inflammatory cytokines, we may find IL6, IP-10, MIP1 alfa, and beta as well as MCP, which bind in turn to monocytes, macrophages, and T cells, promoting the production of gamma interferon. This creates a cycle of persistent and sustained inflammation. Pathogen-associated molecular patterns such as viral RNA and danger-associated molecular patterns, including adenosine triphosphate and DNA are detected by pattern recognition receptors, alveolar epithelial cells and alveolar macrophages. In turn, this provokes the propagation of local inflammation, involved in the increased secretion of IL6, IL10, gamma interferon, and MCP. Other mediators or inflammatory response observed in Coronavirus disease (COVID-19) are IL2, IL7, IL10, G- CSF, TNF, and CD14 + CD16 + monocytes. The regulation of the secretion of pro-inflammatory cytokines such as IL1 beta and IL8 is regulated by the NLRP3 inflammasome, creating reactive oxygen species and viroporins, pore forming viral proteins, which modify cellular membranes9.
As previously commented in clinical and laboratorial terms, C reactive protein (CRP), procalcitonin, erythrocyte sedimentation rate, serum amyloid protein, IL6, ferritin, and fibrinogen have been evaluated in the development of infection by COVID - 19 and as markers of systemic inflammation and disease severity and progression. Low levels of procalcitonin with high levels of CPR are correlated in this entity, as well as higher levels of IL6 were reported in people who died from this disease10. Nonetheless, PCT an procalcitonin levels may be higher in patients with severe SARS-COV 2 infection. In relation to this, CD14 + CD 16+ monocytes activated by granulocyte - marcrophage colony stimulating factor (GM - CSF) secrete more IL6 than other pro-inflammatory markers. There exists a heterogeneous gamma of laboratory results, as specific levels may depend on secondary infections, variability in laboratory levels, and differences with those reported worldwide, whether it be technique or standard cutoff value, as well as bias in publications depending on the county or region affected. Another marker for disregulation of the immune system, which permits the development of viral hyper-inflammation is the neutrophil/lymphocyte Ratio (NLR) and the lymphocyte/platelet Ratio (LPR). It is theorized that these indexes may help predict clinical severity in patients with COVID 19, specifically, expecting NLR levels to increase significantly while LPR levels reduce, reflecting, in turn, an acute inflammatory process and possibly a worse prognosis11.
Evidence exists which suggests that an elevation of LDH above 350 IU may be considered an important marker for the severity of infection by COVID 19. Furthermore, a negative impact is assumed regarding the survival with patients with pneumonia. This was observed in patients who had an AH1N1 influenza pneumonia, infection by pneumocystis jirovecci and bacterial pneumonia. In the specific context of patients with SARS-COV 2 pneumonia, an increment in LDH levels has been observed as a possible predictor of tissue damage. Information is needed to establish this biomarker as a prognostic tool in patients with severe disease2,5,6.
Materials and methods
A retrospective cohort study was conducted including severe COVID-19 hospitalized patients in the American British Cowdray (ABC) Medical Center. The infection was confirmed by oropharyngeal RT-PCR to detect SARS-CoV-2. All patients had severe COVID-19 disease and required supplementary oxygen. Studied biomarkers include glucose, albumin, neutrophil/lymphocyte ratio (NLR), LPR, CRP, procalcitonin, D Dimer, LDH, ferritin, 25 - OH - Vitamin D, and IL6.
Clinical and laboratorial variables were collected on the patients' admission. Electronic medical records were assessed to evaluate disease progression, which was defined as patients who needed vasoactive amines, invasive, or non-invasive mechanical ventilation or death.
Data were analyzed using the statistical program SPSS version 22 (Chicago, IL). For variables which had a normal distribution, the mean and standard deviation were used and for free distribution variables, the median, and interquartile range. To contrast the differences which were found between the groups with or without complications, a T student test or a Mann-Whitney U-test were used for quantitative variables or normal distribution or free distribution, respectively. For qualitative variables, a Chi-square test was applied. For determination of the best cutoff value for each variable, a receiver operating characteristic (ROC) curve was used. With regard to the primary outcome, a Chi-square test and relative risk (RR) were utilized.
Results
We report 46 patients hospitalized with SARS-COV 2 severe infection confirmed by RT-PCR in the ABC Medical Center in Mexico City. The mean age was 51 years SD (16.7), the majority of whom were male 30 (65%), 38 (82%) being overweight or obese, 10 (21%) had type 2 diabetes mellitus, and 15 (32%) had hypertension (Table 1).
Table 1 Basal characteristics of patients on entry to hospitalization
| Characteristics | Value |
|---|---|
| Age | 51.5 (16.7) |
| Gender | Men 30 (65.2%) Women 16 (34.8%) |
| Cancer | 2 (4.3%) |
| Type 2 diabetes mellitus | 10 (21.7%) |
| Hypertension | 15 (32.6%) |
| Body mass index (BMI) (Kg/m2) | 27.4 (25.8-30.8) |
| Overweight or obesity BMI > 25 Kg/m2 | 38 (82.6%) |
| Glucose (mg/dL) | 111 (98-129) |
| Leukocytes (× 109/L) | 7.2 ( 4.6-11.2) |
| Neutrophils (× 109/L) | 6.1 ( 3.9-12.2) |
| Lymphocytes (× 109/L) | 0.96 (0.7-1.3) |
| Hemoglobin (g/dL) | 14.7 (13.5-16.1) |
| Platelets (109/L) | 222 (146-280) |
| Lactate (mmol/L) | 1.4 (1.0-1.9) |
| Creatinine (mg/dL) | 0.9 (0.7-1.0) |
| Albumin (g/dL) | 3.6 (0.4) |
| 25-0H-Vitamine D (ng/dL) | 15.5 (12.9-19.6) |
| Lactic dehydrogenase (LDH) (UI/L) | 310 (215-418) |
| D Dimer (mcg/L) | 841 (564-1384) |
| Procalcitonin (ng/mL) | 0.2 (0.09-0.63) |
| C reactive protein (mg/L) | 11.9 (5.5-21.9) |
| Ferritin (mcg/L) | 799 (420-1522) |
| IL-6 (pg/mL) | 73.8 (33-202) |
| Neutrophil/lymphocyte Ratio (NLR) | 6.7 (4.2-12.7) |
| Lymphocyte/platelet Ratio (LPR) | 221 (139-319) |
For normal distribution mean and standard deviation were used and for free distribution the median and interquartile range.
The median hospitalization was 9 days (interquartile range of 6-21 days). Patients who presented disease progression were 23 (50%). Disease progression includes death of 4 patients (8.7%), invasive mechanical ventilation in 20 patients (43.5%), non-invasive ventilation in 12 patients (26.1%), and use of vasoactive amines in 17 patients (37%). The median days until death were 18. During treatment, steroids were used in 16 (34%) of the patients. Other treatments used were antibiotics, antivirals, and tocilizumab. An analysis by treatment type was not performed. While contrasting the patients' basal characteristics with the presence of complications, age, and levels of LDH stand out among the studied variables (Table 2).
Table 2 Basal characteristics of patients on entry to hospitalization according to the presence of in-hospital complications
| Characteristics | With complications (n = 23) | Without complications (n = 23) | p value |
|---|---|---|---|
| Age (Media) | 62 | 49 | 0.004 |
| Gender (Men) | 16 (69.6%) | 14 (60.9%) | 0.53 |
| Cancer | 2 (8.7%) | 0 | 0.48 |
| Type 2 diabetes mellitus | 7 (30.4%) | 3 (13%) | 0.28 |
| Hypertension | 10 (43.5%) | 5 (21.7%) | 0.20 |
| BMI (kg/m2) (Mean) | 27.7 | 27.7 | 1.0 |
| Overweight or obesity (Mean) | 19 (82.6%) | 19 (82.6%) | 0.66 |
| Glucose (mg/dL) | 183 | 104 | 0.14 |
| Leucocytes (× 109/L) (Mean) | 8.6 | 6.7 | 0.37 |
| Neutrophils (× 109/L) (Mean) | 6.2 | 6.0 | 0.82 |
| Lymphocytes (× 109/L) (Mean) | 0.9 | 1.0 | 0.96 |
| Hemoglobin (g/dL) (Mean) | 14.6 | 14.9 | 0.84 |
| Platelets (109/L) (Mean) | 220 | 224 | 0.90 |
| Lactate (mmol/L) (Mean) | 1.7 | 1.0 | 0.39 |
| Creatinine (mg/dL) (Mean) | 0.91 | 0.89 | 0.62 |
| Albumin (g/dL) | 3.5 | 3.6 | 0.46 |
| 25-0H-Vitamine D (ng/dL) (Mean) | 14.9 | 15.5 | 0.31 |
| LDH (UI/L) (Mean) | 386 | 284 | 0.01 |
| D Dimer (mcg/L) (Mean) | 888 | 766 | 0.35 |
| Procalcitonin (ng/mL) (Mean) | 0.25 | 0.13 | 0.37 |
| C reactive protein (mg/L) (Mean) | 13.2 | 10.5 | 0.66 |
| Ferritin (mcg/L) (Mean) | 872 | 741 | 0.18 |
| IL-6 (pg/mL) (Mean) | 101 | 51 | 0.08 |
| PNR (Mean) | 6.91 | 6.35 | 0.78 |
| LPR (Mean) | 187 | 229 | 0.55 |
For normal distribution mean and standard deviation were used and for free distribution the median and interquartile range
The variables of significant association with the primary outcome comprised of death, use of vasoactive amines or mechanical ventilation include age, glucose, and the LDH levels on hospital admission. The cutoff levels were age above 50 years, glucose levels above 126 mg/dL, and LDH levels above 410 UI/L (Table 3). ROC curves were created for each of the studied variables to determine the best cutoff point for their associations to complications registered during their hospitalization. For age, an area below the curve of 0.72 (0.57-0.87, p = 0.008) was established, for the levels of LDH, 0.70 (0.55-0.85, p = 0.01), and for glucose 0.81 (0.68-0.93, p ≤ 0.001) (Fig. 1).
Table 3 Relative risk for death, use of vasoactive amines, or use of mechanical ventilation
| Variable | Relative risk | Interval | p value |
|---|---|---|---|
| Age > 50 years | 4.5 | 1.2-16.9 | 0.003 |
| Glucose > 126 mg/dL | 2.75 | 1.64-4.58 | 0.001 |
| Albumin < 3.5 g/dL | 1.25 | 0.6-2.2 | 0.46 |
| Procalcitonin > 2 (ng/mL) | 0.71 | 0.2-1.8 | 0.69 |
| C reactive protein > (5mg/L) | 1.31 | 0.58-2.99 | 0.72 |
| Ferritin > 1500 (mcg/L) | 1.82 | 1.08-3.05 | 0.09 |
| D Dimer > 1000 (mcg/L) | 1.19 | 0.67-2.12 | 0.54 |
| LDH > 410 (UI/L) | 2.04 | 1.25-3.34 | 0.03 |
| IL-6 > 50 (pg/mL) | 1.21 | 0.63-2.33 | 0.53 |
| LNR > 5 | 1.0 | 0.54-1.8 | 1.0 |
| LNR > 3 | 2.2 | 0.64-7.59 | 0.12 |
| LPR > 300 | 1,35 | 0.76-2.39 | 0.51 |
| LPR > 150 | 0.73 | 0.41-1.30 | 0.32 |
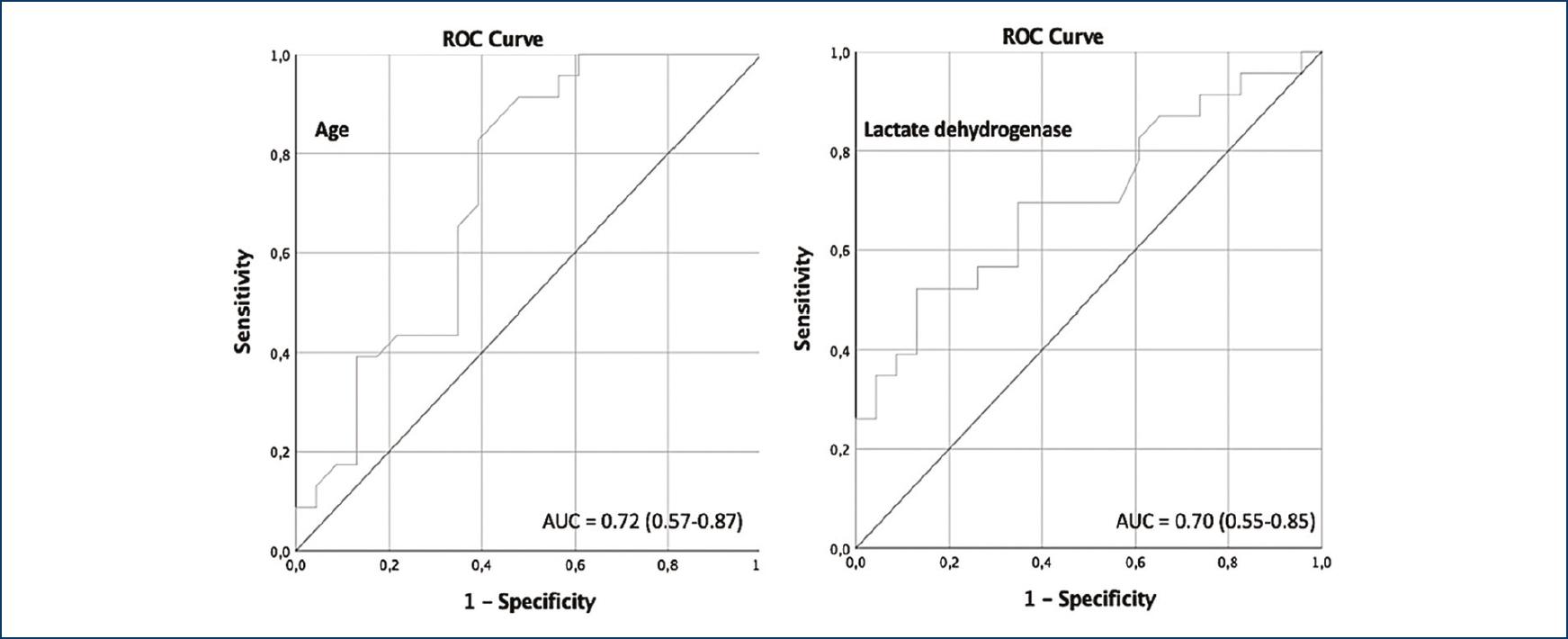
Figure 1 Receiver operating characteristic curve comprising age, glucose, and lactic dehydrogenase as markers of primary outcome.
No association was found between the primary outcome and type 2 diabetes mellitus (RR 1.57, 0.91-2.71, p = 0.28) or hypertension (RR 1.59, 0.92-2.74, p = 0.20).
The primary outcome was broken down and a sub-analysis was performed between each variable. Statistical significance was found between invasive mechanical ventilation in patients above 50 years of age (RR 3.93, 1.05-14.72, p = 0.01), glucose levels above 126 mg/dL (RR 3.05, 1.65-5.63, p = 0.001), and LDH levels above 410 UI/L (RR 2.12, 1.18-3.80, p = 0.03). In addition, vasoactive amines use was associated with age above 50 years (RR 7.0, 1.02-47.75, p = 0.007) and glucose levels above 126 mg/dL (RR 3.53, 1.69-7.36, p = 0.002).
Discussion
In this retrospective cohort study, we show the characteristics and clinical outcomes of 46 patients hospitalized in the ABC Medical Center infected with COVID-19. Although many clinical characteristics are similar to those reported in the previous studies12, the fact that this has been reported in different regions of the world underlines the importance of studying the different clinical features present in our population, and with this, biomarkers which may help predict a worse outcome. It may be observed that almost the double of patients who were admitted with severe COVID-19 pneumonia were men (65.5% vs. 34.8%), as has been noted in national statistics. This differs with reports from other countries, in which the reported incidence is similar in both genders (men 59% and women 41%12-14), as well as male mortality, documented to be almost 2.4 times more in women, regardless of age or comorbidities15. There has been speculation that an explanation to this phenomenon is the protective role estrogens play in severe infection, angiotensin-2 receptors, which have been described as the entry point of the virus, and the anti-inflammatory role the angiotensin-converting enzyme has. Specifically, its gene is located in the X chromosome, which may be correlated to the higher levels of this enzyme in women and as such, an additional protective factor16.
Among the comorbidities reports in our population, it is important to note there exists a higher proportion of patients with hypertension (32.6%) in contrast to national statistics (19.7%) and world statistics (6%). Regarding type two diabetes mellitus; in our study, there was a prevalence of 21.7%, once again higher in relation to national statistics (16.5%), and world statistics (6.3%)13,16. It is, therefore, interesting to note that this increase in the prevalence of comorbidities in our population did not translate into a higher rate of statistically significant complications. The previous reports in patients with pulmonary infections have shown a correlation with the increase of LDH and elevation in respiratory complications, such as the use of mechanical and non-mechanical ventilation. This is even more prevalent in patients with opportunistic infections or infections by mycobacterium5. In the AH1N1 Influenza epidemic of 2009, 77.8% of patients with LDH levels > 225 U/L had a significant increment in mortality levels17.
While comparing clinical outcomes based on biomarkers, we show the relationship that the increment of LDH and age as factors of poor prognosis and as predictors of complications in hospitalized patients. It has been demonstrated that there is a 6-time increment in the probability of presenting more severe forms of SARC-COV 2 with LDH levels above the standard cutoff value, these patients are 16 times more likely to die from the disease18, with a sensibility of up to 100% and a specificity of 86.67% for LDH levels higher than 283 U/L18-20. It was of critical importance to evaluate these findings and their applicability in our population, as they have demographically distinct characteristics. This was reproduced in our study; therefore, we must deduce that patients with these variables must be monitored in a closer fashion20. It is also relevant to note that there exist multiple factors that may elevate levels of LDH; thus, this variable must be considered in the individual context of the patient to be able to use it to establish a prognostic profile. Glucose level was useful to predict disease progression not considering if the patients were previously diabetic and above other biomarkers, this result is consistent with other cohorts21. Biomarkers such as IL-6, the NLR, and the LPR did not demonstrate statistical significance in our analysis toward the creation of a prognostic profile and the demonstration of an increment in mortality nor complications. Ferritin levels above 1500 tend to be significant, like other studies, it is possible this may be related to the size of the study. Recent reports have demonstrated a direct relationship between age and an increment in morbidity and mortality rates14,22. This correlates directly with our study, showing that patients over 50 years of age were found to have a higher risk of complications included in the primary outcome, such as mechanical ventilation and death. This was not reproduced in patients with hypertension, type two diabetes mellitus, obesity, chronic renal, or hepatic disease. This also may be explained by the sample size and the small number of patients with these comorbidities seen in the ABC Medical Center, as well as how, in general, the patients in our population who have these diseases maintain them under control with medical treatment, which lowers the possibility of presenting complications in itself.
The limitations of our study are: a small sample size, the evolution days before hospital admission were not taken into account, the biomarkers were analyzed in a single moment, an analysis by treatment type was not performed, and the design does not include a comparative arm with pneumonia due to other etiologies.
The use of biomarkers, specifically as a prognostic profile is of increasing utility in daily clinical practice as a way to predict the evolution of an ill patient and enable intervention in a timely fashion to avoid complications. This is particularly important in a country with limited resources, both in relation to the attention critically ill patients receive and medications that are available to them. To be able to predict complications in patients may also help in relieving health related costs. This study permits the identification of biomarkers to predict such complications and opens a pathway onto a new generation of hypothesis which may be of use toward medical response in this pandemic.
Conclusions
Patients with SARS-COV 2 severe infection, age over 50 years, glucose above 126 mg/dl, and LDH level above 410 IU were associated with disease progression (death, mechanical, and non-mechanical ventilation or use of vasoactive amines). Ferritin levels above 1500 tend to be significant. Other inflammatory parameters such as NLR and LPR did not show a statistical significant association as predictors of severe disease. Conflicts of interest
The authors declare that does not exist any conflicts of interest











 nueva página del texto (beta)
nueva página del texto (beta)


