Introduction
The global pandemic of 2020 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV)-2, which generates the disease named coronavirus disease (COVID)-19, has challenged all the sanitary systems of the world since its apparition in Wuhan, China. Until July 30, 2020, it has been reported more than 17 million of positive cases and more than 667 thousand deaths in more than 200 countries. This virus is similar than the one described on SARS-CoV and Middle East respiratory syndrome-related coronavirus epidemics. Animals are intermediate reservoirs between humans1,2.
We have observed since January of 2020 that the information has changed through the days and weeks. Therefore, what we write today could not be valid tomorrow.
We present a group of ambulatory patients treated by our team, considering the experience acquired by physicians from China, France, Spain, and some other countries of Europe. We revised several treatments and alterations present in this disease3.
Clinical cases
We present a series of cases treated on an outpatient basis with COVID-19 clinical data. Almost all of them had a real-time polymerase chain reaction (PRC) for detecting SARS-CoV-2. It is a group of 371 consecutive patients attended in an outpatient basis from March 17, 2020, to May 31, 2020. Ages varied from 17 to 78 years of age with a mean of 42 years. There were 119 women and 252 men (Table 1).
Table 1 Demographic features of 371 COVID-19 patients treated with ambulatory management
| Demographic features | |
|---|---|
| Gender | |
| Women | ]119 |
| Men | 252 |
| Age | |
| Age interval | 17 a 78 |
| Mean | 42 |
| Mexican state of precedence | |
| CDMX | 188 |
| Estado de México | 126 |
| Morelos | 11 |
| Veracruz | 11 |
| Guerrero | 9 |
| Sinaloa | 9 |
| Puebla | 6 |
| Guanajuato | 3 |
| Oaxaca | 3 |
| Jalisco | 2 |
| Sonora | 2 |
| Chiapas | 1 |
| Reference | |
| Other patients | 85 |
| Other physicians | 15 |
| Method of interview | |
| Physical consultation | 72% |
| Call (Telephone or Video call) | 28% |
| Travel to another country | |
| Spain | 8 |
| United States of America | 8 |
| China | 2 |
| Risk factors | |
| Under the age of 60 | 68% |
| Obesity | 65% |
| Diabetes | 42% |
| Older than 60 | 32% |
| Systemic arterial hypertension | 26% |
| Chronic kidney disease | 6% |
All the patients that assisted to consultation referred a sense of general malaise, asthenia and adynamia (100%); temperature above 37°C (80%); chills (76%); mild or severe headache (74%); sore throat (72%); dry cough (70%); sore muscles (70%); ageusia and anosmia, verified using a special set of odors and flavors (45%); and diarrheic syndrome (10%) (Fig. 1).
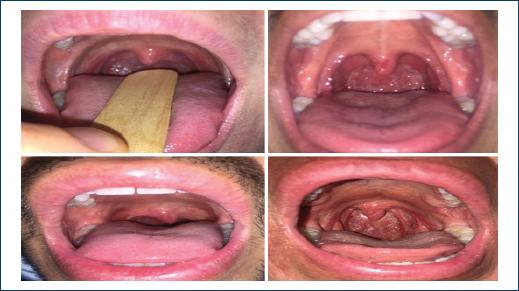
On physical examination, we identified conjunctivitis (54%); pharyngeal redness (72%); decrease of breathing movements (44%); sweaty skin, temperature above 37°C, and oxygen saturation below 90% but > 83% (20%); fine-crackling rales (26%); abdominal pain (23%); expiratory wheeze (15%); and livedo reticularis (10%). A special finding that we want to report is that we noted hyperemia over the uvula with a vascular increase in more than 50% of the patients with COVID-19 (Figs. 1 and 2).
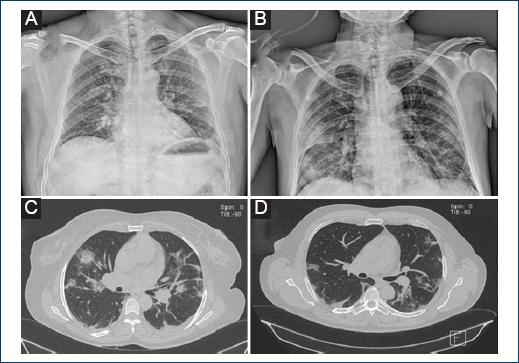
Figure 2 A: chest X-ray film with bilateral patchy and ground-glass opacities. B: chest film with bilateral, reticular, patchy, and ground-glass opacities and intercisural pleural effusion. C: simple thorax tomography with ground-glass opacities, some of them patchy, bilateral, and some of them triangular with inner vertex and external base. D: thorax tomography with a pulmonary window showing ground-glass opacities, patchy, triangular-shaped with external base and inner vertex.
Chest X-ray films were found normal in 6% of the cases, ground-glass opacities in 73%. Simple computer tomography was done in the same patients, finding sub segmentary images, ground-glass opacities, and consolidated images inside the ground-glass opacities in some of them. All the lesions were bilateral and had several sizes; some images had triangle shapes with internal vertex and external base. Minimum pleural effusion was found in 22% of the patients (Fig. 2).
Rapid influenza and COVID-19 tests were carried out in 174 of 371 patients, resulting negative for influenza in 169 cases. Three hundred and twenty COVID-19 tests were positive and 48 negative. All COVID-19 negative test patients had a clinical course compatible with this disease. Three patients were not tested for COVID-19 because they did not want to wait 10 days without treatment until they had the results.
The most important findings in laboratory tests were leucopenia from 2.5 to 3.4 × 10e3/uL, neutrophilia and initial lymphopenia and elevated C-reactive protein (CRP) in 318 patients. Ferritin levels were normal in 27 patients and elevated in 310; procalcitonin levels were slightly elevated in 41 cases; D-dimer level was > 500 ug/L in 152 cases.
Oral temperature > 37°C was present in 330 patients and between 36 and 37° in 41 patients at the time of physical examination. Only eight patients of the series, hospitalized were intubated. Two patients with the chronic obstructive pulmonary disease died and the rest recovered.
Today we know that there is no effective therapy for this SARS-CoV-2; however, we have adopted the experience that China, Korea, Italy, Spain, and France have accumulated in the management of these patients. We analyzed preliminary communications of the use of drugs against SARS-CoV-2 with the analysis of the physiopathology that initially was unknown; the FDA has not approved many drugs due to the lack of clinical essays1,2,4,5.
Based on the information above, the first four cases were treated with oseltamivir 75 mg BID during 10 days and Clarithromycin 500 mg BID for 7 days. These patients had symptoms compatible with COVID-19 and had a test, but the waiting time for receiving the results used to last approximately 10 days. We did not consider prescription of remdesivir or lopinavir/ritonavir because of the high price and low availability. Up to March 15, 2020, we considered the combination of azithromycin 500 mg once per day during 7 days and hydroxychloroquine sulfate 400 mg initially and then 200 mg BID for 7 days. We also considered stimulating the action of T lymphocytes using ivermectin (IVM), based in the management of the alterations observed in the disease (Fig. 3 and Table 2)6-10.
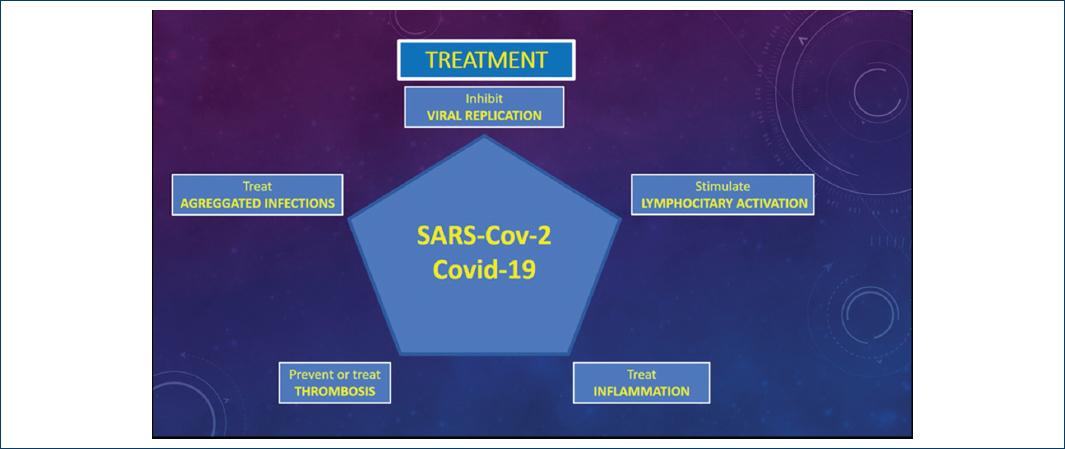
Figure 3 Pentagon proposes the five most important aspects of managing patients with coronavirus disease-19.
Table 2 Treatment scheme used in 371 patients with COVID-19
| Treatment scheme used | |
|---|---|
| Hydroxychloroquine, azithromycin, ivermectin, famotidine, and zinc | 78% |
| Oseltamivir, ivermectin, azithromycin, and famotidine | 22% |
| Amoxicillin/clavulanate | 18% |
| Prednisone | 60% |
| Drugs used in hospitalized patients | |
| Tocilizumab | 11 |
| Dexametasona | patients 8 patients |
All these schemes were given according to the current literature reported in China, Australia, and Europe.
All the cases, in which we prescribed hydroxychloroquine sulfate combined with azithromycin, underwent an electrocardiogram to evaluate the QT interval and calculating the corrected QT interval using Bazett's formula: results of dividing the value of the QT interval over the square root of the RR interval. The decision of being able to administer the drug was made only if the value was favorable11-18.
We indicated half of a milligram per kilogram of prednisone per day from day 5 of treatment, during 20 days, based on their radiological images, and if the dyspnea persisted, evaluating the reduction of the doses gradually according to their radiological evolution.
Of the group studied, 158 patients coursed with hypertension and received angiotensin-converting enzyme inhibitors (ACE inhibitors) without suspending their treatment according to the reports in the literature1. All the patients studied in this series, evolved favorably. Two hundred seventy-four cases were SARS-CoV-2 tested after 10 days of initiating treatment, resulting positive only in two cases that required secondary treatment.
At follow-up, 127 patients persisted with an oral temperature between 37 and 37.3°C and normalized after 21 days. The remaining symptoms, such as headache, disappeared after 18 days of treatment. Five patients were admitted to the intensive care unit due to respiratory insufficiency and needed intubation. They had elevated D-dimer, Type B atrial natriuretic peptide, erythrocyte sedimentation rate, ferritin, lactic dehydrogenase, CRP, and procalcitonin.
Two patients that received hydroxychloroquine sulfate and azithromycin reported long QT syndrome and needed suspension only of azithromycin but completed treatment only with hydroxychloroquine. Alterations in chest X-ray films normalized in 345 cases and the rest is currently in resolution or formation of fibrosis in certain volumes of the lungs. Two cases formed minimal pleural effusion. In the cases where the SARS-CoV2 PRC test resulted negative, but the patients presented the compatible clinical course, we prescribed the treatment scheme, trying to contribute to decrease the evolution of the disease, as done in other countries. Follow-up was carried out in all patients with phone calls, up to 3 times a day. We asked the patients to report oximetry readings in each call. We found a way to send home a radiologist technician to take chest X-ray films as needed in many of the patients. All the patients had access to means of communication and private laboratory services.
Discussion
The most important aspect of our discussion is that, of the PCR negative cases that we have treated, almost 50% have symptoms. Given the severity of these patients or the presence of symptoms, although they had only a few, we administered treatment, observing a non-progressive disease to greater severity. We noted the gradual disappearance of the clinical and radiological signs and symptoms. The presence of a negative test is not conclusive1,2,19; however, positive test in patients with clinical history supports the diagnosis. The problem that we have detected is the time that the results of the tests take, with the urgent need to start treatment, as we managed the patients in the present report.
Real-time PCR test for detecting SARS-CoV-2 RNA specific sequences is the most valid way to detect infected patients, being the most sensitive test available nowadays. In our country, it is difficult to know the most accurate number of cases since our leaders have decided to use the Sentinel model for detecting positive cases, leading us to a distant real number of cases detected. Using this model, we can only have partial information of the total number of cases, resulting in the wrong decisions. The experience of other countries that makes a greater number of tests leads to decisions with better basis1,2,19.
The measurement of oxygen saturation orientates the clinician about the consequences of lung alterations; therefore, it is necessary on decision-making. Oximetry allows monitoring the patient from home and translates one of the best parameters to evaluate the disease. We can also monitor temperature, dyspnea, and general malaise. We can revise the patient from home and indicate laboratory and image studies too1,2,13,14,20,21.
The signs found over the uvula have been described along with other ones in the skin and teguments and most be considered when exploring the patients.
Ground-glass opacities or patchy images seen, both in radiographic images and simple tomography, called our attention due to the observed differences in the studies taken in the patients during the influenza AH1N1 pandemic1-5,20.
We recommend oseltamivir especially in those cases with a clinical course that has symptoms similar than flu disease, and even though its use is still currently discussed, we recommend to prescribe it since it has been described the coexistence of influenza and SARS-CoV-22.
Prescription of hydroxychloroquine/azithromycin is very useful, and we suggest to take an electrocardiogram trace paying attention on DII and V5 electrodes to evaluate the QT interval and subsequently, correct the QT interval using the Bazett's formula that results of dividing the value of QT over the square root of the RR interval11. Nowadays, and after considering several references, we propose the management showed in figure 3 and table 2, as long as considering CURB-65 severity scale (Table 3)8. Remdesivir can be prescribed since there are several series that support its use; however, the cost could not be accessible to everyone1.
Table 3 CURB-65 score for evaluating the severity of COVID-19 patients, adapted from community-acquired pneumonia severity score
| 1. C: Confusion (1 point) | ||
| 2. U: Urea/BUN > 19 mg/dL (1 point) | ||
| 3. R: Respiratory rate (1 point) | ||
| 4. B: Blood pressure ≤ 90-mmHg or Diastolic ≤ 60-mmHg (1 point) | ||
| 5. 65: Older than 65 (1 point) | ||
| Score CURB-65 | Mortality (%) | Recommendation |
| 0 points | 0.6 | Low risk; consider ambulatory treatment |
| 1 point | 2.7 | |
| 2 points | 6.8 | Moderate risk: short hospitalization or ambulatory treatment with strict follow-up |
| 3 points | 14.0 | Severe pneumonia: hospitalization and consider intensive care admission |
| 4 o 5 points | 27.8 | |
There are not evidences that support the suspension of ACE inhibitors.
The use of corticosteroids is controversial in this and other infectious diseases that affect the lungs; however, it is difficult to establish the best moment of its use21-24. In patients with severe pneumonitis due to SARS-CoV-2, tocilizumab combined with steroids could be beneficial since the patients with COVID-19 have elevated levels of pro-inflammatory cytokines such as interleukin 1 and 6 (IL-1, IL-6).
IVM is an intra-intestinal and extra-intestinal, anti-parasite drug used in humans and animals. However, since 1991, Blakely and Roisseaux made an essay where the effect of IVM over the increase in the response of T-cell dependent antibodies was evaluated. The function of T lymphocytes improved as long as inhibition of viral replication and its possible application in different degrees of immunosuppression5-10,23,24.
It is known that inflammation causes hypersecretion of ferritin. SARS-CoV-2 virus has a tropism for the porphyrin group of hemoglobin and can destroy or denaturalize it, bringing consequently an incapacity of the hemoglobin to bind oxygen at the lungs and liberate it in the cells or in the periphery. If this phenomenon remains in the lungs, an intra-alveolar diffuse hemorrhage with severe blockage of the diffusion of oxygen from the alveoli to the lung capillaries can occur5.
There are several clinical essays and protocols worldwide recently in search of different treatments for different clinical stages of COVID-19. Some of them with the intention of improving the oxygenation in intubated patients. There are proposals for using nebulized fibrinolytics such as nebulized tissue plasminogen activator or nebulized Dornase alfa, but they are expensive and with low availability. In addition, a protocol considers the use of nebulized heparin combined with acetylcysteine. The theoretical fundament proposes that SARS-CoV-2 has a spike-like protein on its surface that binds heparin and therefore prevents its replication.
There are multiple causes and pathophysiological mechanisms. The approach requires medical equipment and multidisciplinary health professionals with a personalized focus in the diagnosis and treatment of the COVID-19 patient (Fig. 3)5,20.
Reviewing the literature, in autopsies made in 15 patients that died because of influenza in 2009, the most relevant findings were lymphocyte infiltration predominantly in the interstitium, pneumocyte type II hyperplasia with hyaline membranes that obstruct the diffusion of gases, mucus plugs that obstruct the bronchioles lumen and acini3. In autopsies performed on deceased patients for COVID-19, scarce or absent lymphocytary infiltrate was found in the interstice. There was a prevalence of underlying infiltrate in middle lumen vessels, diffuse alveolar damage with fibrin in the alveolar spaces, minimal inflammation, and squamous metaplasia. Attention was brought to the absence of mucus plugs in bronchi or acini, as long as perivascular inflammatory infiltrates in the median lumen vessels, where some microthrombi were found. This supports the theory that some groups argue that patients died because of heart failure and multiple emboli in small lumen vessels. Patients with COVID-19 have a greater risk of venous thromboembolic disease (VTED).
Pregnant women are part of a group in enhanced risk of VTED, in the case of infection for COVID-19, require a more emphatic evaluation in the pregnancy and puerperium1,24-30.
One of the questions that remain unanswered is: Why do some of the patients produce a lot of inflammation and some others not? There is not much mentioned about it, but we will have to analyze what individual aspects have influence over inflammation.
We believe that it is important to activate the health virtuous quadrangle, where public and private institutions, government, and society interact in favor of the health and contribute to the best management of the disease17.
The pathogenic power of the virus and the alterations that causes can be summarized in five types: 1. increase of viral replication; 2. aggregated infection; 3. development of thrombi in the territory of the pulmonary artery; 4. pulmonary inflammation; and 5. decrease of the lymphocytary function. Therefore, the treatment most be directed to inhibit viral replication with its possible viral destruction; treat the aggregated infection; treat and prevent the development of thrombosis in the pulmonary vascular bed; decrease lung inflammation; and reactivate lymphocyte function. These points above summarize broadly the effects that cause SARS-CoV-2 and being so diverse; the management has to be with more than one drug (Fig. 3).
In a future study, we will separate the patients in terms of different treatments to evaluate particular results. Since no specific treatment is available today for SARS-CoV-2, and due to the severity of the disease, ethical committees must study any prescribed drug in research protocols, peer reviewed, and revised. We know that the treatment that we have proposed is useful to treat the alterations that the virus causes but does not eliminate the virus.
In this disease, what is valid today tomorrow perhaps will not, but what we have today is the only thing we have.











 nova página do texto(beta)
nova página do texto(beta)