Introduction
Medullary thyroid carcinoma (MTC) represents a rare tumor of C parafollicular cells, accounting 5-10% of thyroid malignancies, and being responsible ~ 13.4% of thyroid cancer-associated deaths1,2. One quarter of patients with MTC could pertain to the group of hereditary cancer syndromes, with patients showing multifocal, bilateral, and other manifestations3,4. Germinal pathogenic variants of RET proto-oncogene (Rearranged during Transfection) have been associated with genetics disorders, such as multiple endocrine neoplasia type 2 (MEN2), Hirschsprung disease (HSCR), and familial MTC (FMTC)3. MTC has a penetrance of 80-100% in MEN2 and CMTF and due to an autosomal dominant inheritance pattern, having a 50% chance of inheriting the risk of this malignancy to the offspring5.
The RET proto-oncogene contains 21 exons, located on long arm of chromosome 10 (locus 10q11.2); codifying for a tyrosine kinase receptor (protein RET). This protein is mainly expressed in precursor cells of the neural crest and urogenital tract, acting as receptor for ligands such as glial cell-derived neurotrophic factor (GDNF) family. RET protein activation takes place in normal conditions by a complex of coreceptors and ligands that include two groups of proteins: (1) the GNDF family of ligands (GFLs) such as neurturin, artemin, and persephin and (2) the glycosylphosphatidylinositol-anchored GDNF-family α receptors (GFRαs)3,6.
RET is a one-step transmembrane protein, composed of three functional domains: the extracellular ligand-binding region, the transmembrane portion, and the cytoplasmic tyrosine kinase domain. Four cadherin-like and cysteine-rich yuxtamembrane regions conform the extracellular domain. On the other hand, the intracellular domain contains two tyrosine kinase subdomains (TK1 and TK2) which participate in several transduction pathways activation involved in cell survival, proliferation, differentiation, migration, and chemotaxis6,7.
Phenotype correlated to RET proto-oncogene variants seem to depend on codon change rather than type of amino acid substitution. Variants reported in MEN2A and FMTC affect primarily the extracellular cysteine-rich domain, and less frequently the tyrosine kinase domain8. In MEN2A, most germinal RET gene alterations distort codon 634 in around 85% of cases, with Cys634Arg substitution as the most common. On the other hand, FMTC correlates to pathogenic variants on RET-exons 5, 8, 10, 11, 13, 14, 15, and 16. Conversely, MEN2B has been associated to germinal pathogenic changes of the tyrosine kinase subdomain 2, with 95% of patients showing alteration in codon 918, and 5% in codon 8834,6,9-12.
Confirmation of germinal pathogenic variants of RET proto-oncogene can help starting a personalized diagnosis and management and could be a good preventive measure to reduce mortality in non-affected relatives carriers of germinal genetic pathogenic alterations13. Moreover, Latin American populations lack of extensive personalized genetic and molecular tests, conversely to European descendants, limiting genotype-phenotype comparability across diverse populations14.
For this reason, we started - for the 1st time in Peru - in our institution the implementation of molecular analysis of this key proto-oncogene in a subset of Peruvian patients with diagnosis of MTC and suspicion of a hereditary cancer syndrome (MEN2 or FMTC). We identified genetic variants and show the genotype-phenotype correlation in this case series.
Materials and methods
Patients characteristics
We included 21 unrelated consecutive probands with incoming diagnosis of MTC. These patients had at least one evaluation by a medical geneticist in the outpatient consultation or during hospitalization. We collected clinical, surgical, and pathological reports of our case indexes or - if possible - their relatives. For pedigree's elaboration, we follow the standardized nomenclature suggested by the National Society of Genetic Counselors15. Each consultation and evaluation by a clinical geneticist provided to patients and their relatives a detailed information about the molecular test for RET gene. In so doing, the blood withdrawal was obtained after proband's verbal or signed informed consent. Our project was reviewed and approved by the Institution Review Board (IRB) of Instituto Nacional de Enfermedades Neoplasicas (INEN), with IRB code INEN17-68.
Genomic analysis
Genomic DNA was extracted from periferic blood collected into EDTA tubes, using the High Pure polymerase chain reaction (PCR) Template Kit (Roche Diagnostics GmbH, Mannheim, Germany), according to manufacturer's specifications. DNA was quantified with QubitTM Fluorometer (InvitrogenTM, Carlsbad, CA, USA).
A priori selected exons of the RET proto-oncogene were amplified using the PCR technique. Final volume in these experiments was 25 μL, containing: 80-100 ng of DNA template, 0.4 μM of both primers, PCR Buffer 1 ×, 3-4 mM of Mg2, and 0.625 U of the enzyme Platinum Taq DNA Polymerase High Fidelity (Invitrogen). Mg2, dNTPs, and DNA template concentrations were standardized for each exon independently. We used the Veriti thermocycler (LifeTechnologies), with specific primers for exons and exon-intron limit (~25 bp in-deep intronic position)16. The PCR thermal conditions started with a pre-heating cycle at 95°C for 3 min followed by 35 cycles of denaturation step at 95°C for 30 s, annealing at 60-62°C for 30 s, and extension at 68°C for 40 s, and a final extension cycle at 68°C for 7 min.
Bands of PCR products were observed in a 3% agarose gel carried out at 100V for 45 min, dyed with SYBR® Safe for 15 min. PCR products were purified with the PureLinkTM Quick PCR Purification Kit (Invitrogen); quantifying amplicons with fluorometry using QubitTM Fluorometer (InvitrogenTM, Carlsbad, CA, USA).
Bidirectional resequencing was carried out with BigDye Terminator version 3.1 Cycle Sequencing Kit and the ABI PRISM 3500 Genetic Analyzer (Applied Biosystems®, Foster City, CA, USA), using same exon-specific primers as aforementioned and 12-14 ng of final purified amplicon. DNA sequence analyses were performed with SeqScape® v3.0 (Life Technologies).
Variants were referred to cDNA sequence of RET with accession number NM_020975.5, GRCh38.p27. We described variants following nomenclature guidelines of the Human Genome Variation Society (HGVS) site: http://www.hgvs.org/content/guidelines.ClinVar (URL: https://www.ncbi.nlm.nih.gov/clinvar/?term=RET%5Bgene%5D) and ARUP (University of Utah, URL: http://www.arup.utah.edu/database/) databases were revised for searching of germinal RET variants published elsewhere.
Results
From a total of 21 patients (11 women, 10 men) in our case series, we report main clinical and histologic findings, for example, age of MTC diagnosis and RET proto-oncogene analysis results (Table 1). A positive family history of MTC or PTC was found in three (Prob 4, 6, and 17) of our patients, whose RET molecular analysis showed a germinal pathogenic variant (Figs. 1-2). Our group of patients has been diagnosed at a median age of 37 years old (IQR: 32-49), with the youngest aged 7 years old. Three of our patients (Prob 1, 11, and 14) suffered of a mixed thyroid carcinoma (MTC and PTC) synchronically. One of them (Prob 11) shows a RET gene analysis with a variant of unknown significant (VUS) and a concomitant germinal pathogenic variant (c.2370 G > T; p.Leu790Phe) (Fig. 3).
Table 1 Clinical and morphological phenotypes of the 21 index cases presenting at diagnosis with medullary thyroid carcinoma, and a clinical suspicion of multiple endocrine neoplasia type 2 (MEN2) and familial medullary thyroid carcinoma (FMTC)
| Prob | Gender | Age | FH | Diagnosis | Dx age | Bilateral* | Thyroidectomy | Met | Relevant variant | RET -p Pred | Exon | Classification | R. ATA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 25 | No | MTC/PTC | 25 | Yes | Yes (total) | Yes | Non detected | ||||
| 2 | M | 50 | No | MTC | 50 | No (right lob) | no (Nop)** | Yes | Non detected | ||||
| 3 | M | 8 | No | MTC | 7 | Yes | Yes | Yes | Non detected | ||||
| 4 | F | 28 | Yes | MTC | 9 | No (left lob) | Yes (hemi-izq) | Yes | c.1900T > C | p.Cys634Arg | 11 | Pathogenic | C |
| 5 | M | 33 | No | MTC | 33 | No (left lob) | Yes (total) | Yes | Non detected | ||||
| 6 | M | 44 | Yes | MTC | 37 | ? | Yes (total) | Yes | c.1888T > A | p.Cys630Ser | 11 | Pathogenic | B |
| 7 | F | 55 | No? | MTC + PthA | 55 | No (right lob) | Yes (total) | No | Non detected | ||||
| 8 | M | 34 | No | MTC | 34 | No (right lob) | Yes (total) | No | Non detected | ||||
| 9 | M | 47 | No | MTC | 39 | Yes | Yes (right and left lob) | Yes | Non detected | ||||
| 10 | F | 34 | No | MTC | 32 | ? | Yes (total) | Yes | Non detected | ||||
| 11 | F | 50 | No | MTC/PTC | 49 | Yes | Yes (total) | Yes | c.2370 G > T | p.Leu790Phe | 13 | Pathogenic | A |
| c.2371 T > C | p. Tyr791His | 13 | VUS | ||||||||||
| 12 | F | 62 | No | MTC | 62 | No (right lob) | Yes (total) | No | Non detected | ||||
| 13 | F | 49 | No | MTC + HB | 49 | Yes | Yes (right and left lobules) | Yes | c.1859 G > C | p.Cys620Ser | 10 | Pathogenic | B |
| 14 | F | 39 | No | MTC/PTC | 37 | Yes | Yes (total) | Yes | Non detected | ||||
| 15 | M | 39 | No | MTC | 39 | No (left lob) | Yes (total) | Yes | Non detected | ||||
| 16 | F | 23 | No | MTC | 23 | Yes | no (Nop)** | Yes | Non detected | ||||
| 17 | F | 29 | Yes | MTC/HPT/Pheo | 29 | No (right lob) | Yes (total) | ? | c.1900T > C | p.Cys634Arg | 11 | Pathogenic | C |
| 18 | F | 39 | No | MTC | 39 | Yes | Yes (total) | No | c.1900T > G | p.Cys634Gly | 11 | Pathogenic | C |
| 19 | M | 34 | No | MTC | 34 | No (left lob) | Yes (total) | Yes | Non detected | ||||
| 20 | M | 55 | No | MTC | 55 | No (left lob) | Yes (right and left lobules) | No | Non detected | ||||
| 21 | M | 33 | No | MTC | 33 | No (lob ?) | Yes (?) | No | Non detected |
Dx: diagnosis; F: female; FH: family history of cancer; HB: hepatobiliary neoplasm; HPT: hyperparathyroidism; left lob: left thyroid lobule; M: male; Met: metastasis; MTC: medullary thyroid carcinoma; Nop: non-operable tumor; PthA: parathyroid adenoma; Pheo: pheochromocytoma; PTC: papillary thyroid cancer; R. ATA: American Thyroid Association risk category. Prob: proband's pedigree code; RET -p Pred: RET protein prediction; right lob: right thyroid lobule; VUS: variant of unknown significance.

Figure 1 A: pedigree of proband 4. B: electropherogram of proband 4: pathogenic variant c.1900T > C (p.Cys634Arg). Acc: accident; DM: diabetes mellitus; HT: hypertension; ID: intellectual disability; MTC: medullary thyroid carcinoma; PTC: papilar thyroid cancer; Sarc: sarcoma; TD: non-specified thyroid disease.

Figure 2 A: pedigree of proband 6. MTC: medullary thyroid carcinoma, TC: thyroid carcinoma, TG: thyroid goit, Hem: hemophilia. B: electropherogram of proband 6: pathogenic variant c.1888T > A (p.Cys630Ser).
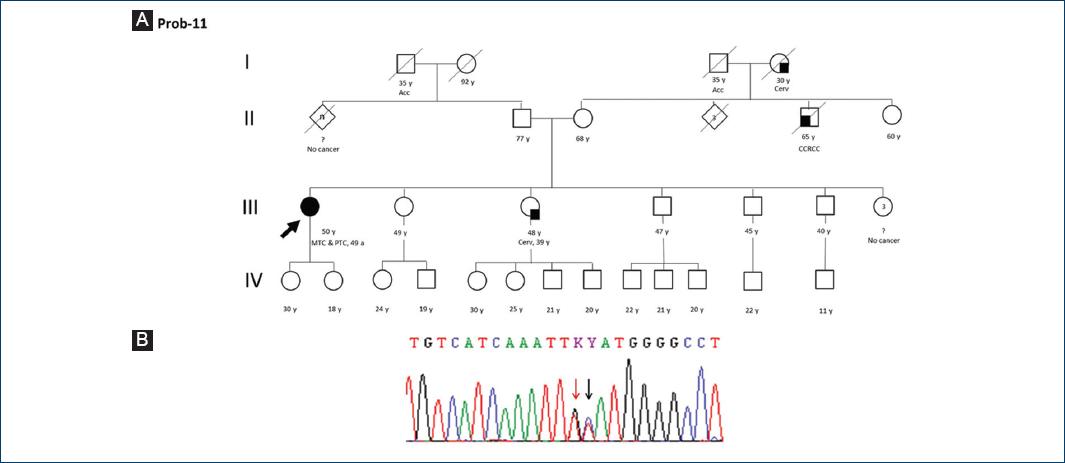
Figure 3 A: pedigree of proband 11. B: electropherogram of proband 11: pathogenic variant c.2370 G > T(p.Leu790Phe) marked with red arrow and a VUS c.2371 T > C (p. Tyr791His) marked with black arrow. Acc: accident; CCRCC: clear cell renal cell carcinoma; Cerv: cervix cancer; MTC: medullary thyroid carcinoma; PTC: papilar thyroid cancer.
Special phenotypic findings were observed in some patients: hepatobiliary neoplasm in proband 13, parathyroid adenoma in proband 7, hyperparathyroidism (HPT), and pheochromocytoma (Pheo) in proband 17. The two latter patients have MEN2A confirmed diagnosis. On the other hand, none of our patients show until date any MEN2B clinical manifestation (i.e., mouth neuromas, marfanoid habitus, and intestinal ganglioneuromatosis).
This cohort shows a characteristic clinical profile according to available data on clinical records. For instance, 8 of 19 patients had bilateral thyroid disease, with most of them presenting metastasis at diagnosis. Thyroidectomy was performed in 90.6% (19/21) of our index cases, being the radical or bilobulated extirpation procedures the preferred for surgeons. The remaining two patients presented inoperable thyroid cancer.
Six of our probands (28.6%) with MTC had a germinal RET pathogenic variant (Prob 4, 6, 11, 13, 17 y 18) identified with Sanger resequencing, confirming the diagnosis of predisposition cancer syndrome related to MTC. Half of these patients (Prob 4, 6 y 17) reported a positive family history of cancer. Furthermore, we found five - in our six index cases - different missense pathogenic variants of the RET proto-oncogene exons: p.Cys620Ser (exon 10), p.Cys630Ser, p.Cys634Gly, p.Cys634Arg (both in exon 11), and p.Leu790Phe (exon 13) (Figs. 1-6). Detected pathogenic variants in the cystein-rich yuxtamembrane domain of the RET protein were found in 83.3% (5/6) of our positive cases - these mutations affect cystein residues of extracellular domain. Most of the genetic changes were attributed to exon 11 (66.6%), and half of our patients with a germinal RET proto-oncogene alteration depict a harmful change at codon position 634 of RET protein.
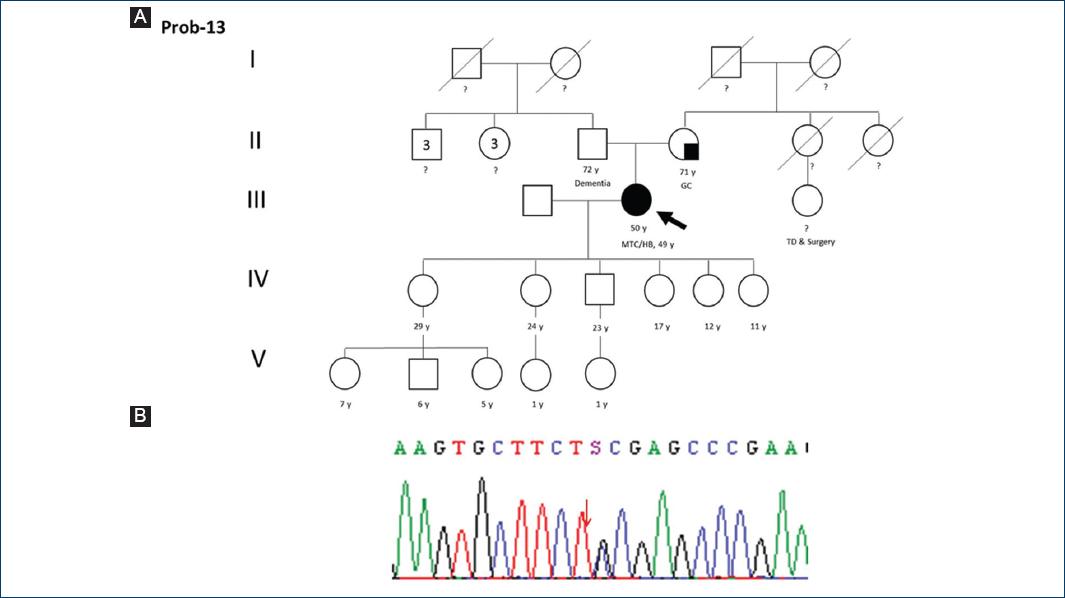
Figure 4 A: pedigree of proband 13. MTC: medullary thyroid carcinoma, GC: gastric cancer, TD: non-specified thyroid disease, HB: hepatobiliary neoplasm. B: electropherogram of proband 13: pathogenic variant c.1859 G > C (p.Cys620Ser).

Figure 5 A: proband 17. MTC: medullary thyroid carcinoma, TC: thyroid carcinoma, HPT: hyperparathyroidism, Pheo=pheochromocytoma. B: electropherogram of proband 17: pathogenic variant c.1900T > C (p.Cys634Arg).

Figure 6 A: proband 18. MTC: medullary thyroid carcinoma. B: electropherogram of proband 18: pathogenic variant c.1900T > G (p.Cys634Gly).
Additional genetic findings help us to better describe our case series. In Proband 11, we detected a concomitant - to the pathogenic variant p.Leu790Phe - VUS on exon 13: c.2371T > C, producing a change on codon 791 (p.Tyr791His). Moreover, benign variants were observed in our patients, such as p.Gly691Ser, p.Leu769Leu, and p.Ser904Ser, with frequencies of 81.0% (17/21), 95.2% (20/21), and 81.0% (17/21), respectively. Most of these nucleotidic changes were found in homozygosis. On the other hand, one of our 21 patients depicted a benign variant (c.2608-24G > A) on intron 14.
Discussion
This report demonstrates high frequency (28.6%) of germinal pathogenic variants in Peruvian patients with MTC and suspicion of MEN2 or FMTC. This finding agrees with the observation that 25% of patients with MTC would carry a pathogenic change on the proto-oncogene RET1,4,5. Moreover, most of our cases with MEN2 or FMTC molecularly corroborated were above 30 years old, differing from other report where patients aged ≤ 30 years depicted higher rates - compared to older than 30 - of germinal pathogenic variants (50% vs. 13.5%, respectively)17.
Germinal variants of RET proto-oncogene associated to MEN2 or FMTC occur due to changes in exon-codificating the cystein-rich and the activable intracellular tyrosine kinase domains of this transmembrane protein6. Phenotypic expression of MEN2 or FMTC seems to correlates with specific changes on RET protein codons10; hence, clinical recommendations depend on type of genetic alteration reported18,19.
Codon 634 of RET protein seems to be affected in 85%6 of cases associated with MEN2A mainly due to a substitution of cystein by arginine (c.1900T > C; p.Cys634Arg), resulting a relevant predictor of Pheo and parathyroid disease20,21. The proband 17 had a diagnosis of MEN2A due to MTC, Pheo, and HPT, presenting the variant c.1900T > C of RET gene, with a strong family history: 2 first-degree, 1 second-degree, and 2 third-degree relatives with MTC or unspecified thyroid carcinoma (Fig. 5). According to ATA4, this index case pertains to group C risk classification, the highest risk group related to MEN2A and FMTC.
Tyrosine specific residues of RET protein which is activated through phosphorilation can help secondary tranducers to continue the intracellular signaling. To date, up to 18 phosphorylation sites have been reported, for example, codon 791 (Tyr791)6,22. One of our patients (Prob 11) carries a pathogenic variant of RET proto-oncogene: c.2370 G > T (p.Leu790Phe) and a VUS c.2371 T > C (p.Tyr791His). Histologic findings in this female patient demonstrated a mixed thyroid carcinoma (MTC and PTC), without oncologic family history. The germinal pathogenic change corresponds to category A following 2009 ATA guideline4. Additional aminoacid substitutions in codon 791, as p.Tyr791Asn and Tyr791Phe, are found in ClinVar named as VUS or with conflicting interpretations of pathogenicity, respectively. The latter also belongs to ATA risk group A, therefore a minor-risk category.
Another relevant genetic change on RET protein has been reported for RET c.1888T > A (p.Cys630Ser) detected in proband 6. This male patient of 44 years old had a strong famility history of thyroid carcinoma: 6 of first-degree, 4 of second-degree, and one of third-degree relatives. We were able to perform germinal molecular analysis of RET proto-oncogene in five relatives. Thus, two MTC-affected relatives (III-23 and IV-3) carry the p.Cys630Ser variant, likewise in two apparently disease-free, first- (IV-15) and third-degree (V-1) relatives - aged 9 and 3 years, respectively (Fig. 2). This variant correspond to ATA risk category B, with prophylactic thyroidectomy recommendation before 5 years old. Although this is a known mutation spot23-26, our detected genetic alteration with a amino acid replace of cysteine by serine, is unique to the best of our knowledge, and this could suggest a Peruvian genetic alteration transmitted trough generation in the Andean region. Further, epidemiological studies would help to confirm this hypothesis.
Ten to fifteen percent of people with diagnosis of MEN2A or FMTC show alteration in codons 609, 611, 618, or 620 in exon 105. However, Hansen et al.27 suggest that exon 10 alteration of RET protein could be more common than previously stated in FMTC. They reported mutations on this exon in 8 of 10 evaluated families. Proband 13 carries a pathogenic variant in codon 620 (p.Cys620Ser) (Fig. 4). She depicts an unusual and ever reported (to our knowledge) metachronic presentation of MTC and hepatic metastasic cholangiocarcinoma.
Asymptomatic patients carrying a germinal pathogenic variant of RET proto-oncogene would benefit from prophylactic thyroidectomy, despite serum calcitonin levels28. Other studies support this surgical approach at appropriate age for disease-free survival outcomes29-32.
We can find current risk classification categories, proposed by health-care institutions through genotype-phenotype correlations. For instance, ATA shows four categories from A to D, according to the risk associated of developing an aggressive MTC at young age4. Thus, the Category D suggests the highest risk, recommending prophylactic thyroidectomy in the 1st year of life. Categories B and C suggest this surgical procedure before 5 years old and at older ages if the asymptomatic patients is ranked in the Category A.
In 2010, The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors published recommendations classifying variants of the RET proto-oncogene in three groups, as follows: level 1, low risk (codons 609, 768, 790, 791, 804, and 891), level two, high risk (codons 611, 618, 620, and 634), and level 3, the highest risk (codons 883, 918, and 922). Recommended age for prophylactic thyroidectomy for these risk groups is as follows: around 5 years old (but < 10 if not performed before), before 5 years old and within the first 6 months of life, respectively33.
This report represents the first case series of Peruvian patients with diagnosis of MTC and with molecular confirmation of germinal RET proto-oncogene variants. The patients were derived to a national neoplastic institute. Moreover, we analyzed relevant exons associated to MEN2 or FMTC, focusing in a priori exons-selected analysis. Nevertheless, most pathogenic variants reported in the literature in patients with these diseases located at RET exons included in our work. On the other hand, one limitation of this report was the difficulty of evaluating punctual pathogenic variants in all proband's relatives. However, we demonstrated in one family (Prob 6) the importance of performing this personalized genetic test for preventive action.
This is the first report of germinal pathogenic variants of RET proto-oncogene found in Peruvian patients with MTC, with some unique findings, demonstrating the importance of molecular analysis for corroborating clinical diagnosis, starting adequate prevention, and including personalized management in countries with limited resources.
Conclusion
We demonstrated a high frequency of pathogenic germline variants of the RET proto-oncogene, half of them being de novo. Furthermore, we found a peculiar genetic alteration in a large family with several affected members, which could be unique to the Peruvian population and could have a founder effect. This manuscript reinforces the importance of developing genomic and personalized medicine in the Peruvian health system.











 nueva página del texto (beta)
nueva página del texto (beta)


