Introduction
Liquid injectable silicone has been in use as a facial cosmetic filler for more than five decades, even though the Food and Drug Administration (FDA) has only approved its use for intraocular injection in cases of retinal detachment and several warnings about this of label use have been published1.
The main complications arising from the use of these materials as facial fillers are granuloma formation and migration of silicone oil.
A siliconoma is a foreign body granulomatous reaction that presents a few days or even years after silicon oil fillers are injected. It usually behaves as recurrent inflammatory attacks with periods of remission between them2,3.
In this paper, we report about a patient with a history of glabellar silicone oil injection that presented with a siliconoma mimicking an acute dacryocystitis.
Case report
A 63-year-old female patient presented with an acute case of pain, swelling, and erythema in her right eye medial canthal area, she had no previous ocular or systemic medical history besides the fact that she has a facial filler injection in the glabella 3 years before her symptoms started, she was diagnosed as an acute dacryocystitis and treated with systemic and topical antibiotics with resolution of her symptoms. Two weeks later, she presented with a recurrence, with fistulation and secretion of white pus-like material, she was treated again with systemic and topical antibiotics and referred for an oculoplastic consultation.
On clinical examination a 5 mm rubbery, non-mobile tumoration was found superior to the right medial canthal angle. No reflux through the lacrimal puncta was found on lacrimal sac massage, a negative fluorescein disappearance test, a positive Jones I test, and no nasolacrimal duct (NLD) obstruction on irrigation was found.
An orbit computed tomography (CT) scan was performed, where a supracantal 6 mm well-defined radio-opaque mass with a 114 Hounsfield unit (HU) density was found (Fig. 1).

Figure 1 Orbital computed tomography showing a 6 mm diameter cystic lesion in the medial supracanthal area with a 114 Hounsfield unit density (arrow).
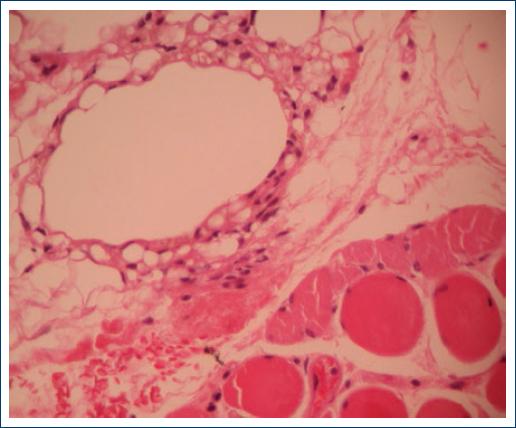
An excisional biopsy was done under local anesthesia, and the histopathology results demonstrated a chronic foreign body granulomatous reaction with numerous optically empty vacuoles that resulted in negative to all the special staining techniques (Figs. 2-3).
Discussion
Liquid silicon was first used as a cosmetic filler in the 1940s decade, with the off label injection of medical-grade silicone oil that was intended for use as a lab material lubricant. Thousands of patients were injected in the US and Europe. Some physicians even mixed the silicone oil with olive oil to induce an even greater fibroblast reaction. In many cases, this injection has disastrous and disfiguring results for the patients4.
In the 1980s decade, Webster et al. described the microdroplet injection technique, which consists of the injection of multiple 0.005-0.01 cc with a 30 g needle for a total of 0.02-0.1 cc of silicone oil injected. The volume augmentation achieved by this technique is based on the fibrosis and encapsulation of these microdroplets. Advocates of silicone oil used as a filler claim that with a proper injection technique and using medical grade purity silicon oil, the procedure is safe and predictable5. Despite this claim, up to date, there are no FDA approved silicone oil-based cosmetic fillers.
The complications have been reported from the use of silicone oil as a filler range from ecchymosis, edema, and erythema, which can happen with any cosmetic filler, up to serious complications such as granuloma6,7, migration of the filler7, cellulitis, ulceration, disfiguring scaring8, pneumonitis, emboli, and death9,10.
In the present clinical case, we found migration and granuloma formation in the medial canthal area 3 years after the filler was injected. The case was misdiagnosed as an acute dacryocystitis but after acute inflammation subsided no NLD obstruction was found, and a tumefaction superior to the lacrimal sac was palpated. In the CT, the tumoration had a density of 114 HU, which is compatible with silicon oil density11; histopathology confirmed that it was certainly a siliconoma and confirmed the migration of the silicon oil filler previously injected.
Conclusions
Undesirable effects from non-approved cosmetic fillers like silicon oil can have serious deleterious consequences for patients.
In cases of a suspected dacryocystitis without any previous history of tearing and a patent lacrimal system after inflammation remission, a detailed medical history and exploration should be done. Imaging techniques are of great value for diagnosis and surgical planning in case a space-occupying lesion is found since almost 55% of lacrimal sac tumors are known to be malignant12.











 text new page (beta)
text new page (beta)