Introduction
At birth, mammals make the transition from aseptic surroundings to a pathogen-filled environment. The gut is constantly exposed to a high antigenic load coming from the diet, commensal, and pathogenic microorganisms. The mucosal epithelial layer of the gut forms the interface between the external and internal environments of the GIT. This area is the site for digestion and absorption of various essential nutrients that, in newborns, allow the passage of nutrients (which generally originate from breastfeeding), for the maturation of cells in different tissues. In addition to this physiological barrier, an immunological barrier is created and maintained by immune cells located in the gut-associated lymphoid tissue (GALT). The development of this immunological barrier is generated as an adaptation response of the newborn to extrauterine life. This barrier is protected by numerous innate defense mechanisms, which operate in close cooperation with adaptive mucosal immunity1.
The mucosa-associated lymphoid tissues make up the largest immune organ in the body, acting at several host-environmental interfaces, including the GIT, the bronchopulmonary, and genitourinary systems. The first contact of the microorganisms that enter the host by oral means is through the GALT, and it constitutes the most extensive and complex part of the immune system.
It is important to note that the adult gut mucosa contains some 80% of the body B-cells2,3 and at least 90% of microorganisms infecting human beings use the mucosa as portals of entry4.
Mucosal immunity provides a first defense line and is most abundantly expressed in the gut. The adaptive immunity of GALT is represented by antigen-presenting cells such as macrophages, dendritic cells, subpopulations of T cells, and subpopulations of B cells. These cells, through the interaction with the antigens, are transformed into plasma cells (PCs), producers of the different immunoglobulins (Igs). Within the Igs, the PCs produce dimeric IgA and pentameric IgM that can be exported through the secretory epithelium to the intestinal lumen2,3. IgA is the most important immunoglobulin in mucous membranes.
It is known that different factors modulate immune responses but, in the digestive tract, breast milk may play an important role in the maturation of the immune response, mainly in relation to GALT5. In this sense, it has been demonstrated that besides nutrients and antibodies, breast milk contains cells6,7, hormones, cytokines, and growth factors8,9 as a proline-rich protein10 and an epidermal growth factor (EGF)11. All of these biologically active factors not only facilitate the development of essential digestive functions but also regulate the maturation of the intestinal mucosal barrier12. In addition to their normal physiological functions, many of these factors have the capacity to stimulate the healing processes in the injured intestinal epithelium and play a role in the maturation of the immune system12. Colostral cells are immunologically reactive and elicit cell-mediated immunity and synthesize immunoglobulins, predominantly those of the IgA class13. A colostral proline-rich protein induces the growth and differentiation of resting B-cells. The capacity of this protein to induce both B-cell growth and differentiation may provide evidence that its central role is the establishment of an immune response in neonates10. EGF is a peptide that produces a variety of biological responses, most of which involve regulation of cell replication, cell movement, and cell survival. In the GIT, EGF not only enhances proliferation and differentiation of epithelial cells but also has significant effects on healing damaged mucosa or on intestinal adaptation after injury11. The amino acid, L-glutamine is also present in the breast milk of the sow, and plays a dual metabolic role in newborn pig enterocytes acting as both a primary metabolic fuel and as an essential source of carbon and nitrogen for biosynthetic processes14. In addition, L-glutamine is important for lymphocyte proliferation and generation of lymphokines15,16. All these factors participate in the maturation of the immune system and in particular of the GALT, and this favors the protection of the individual in the presence of pathogens.
The intestine has defense mechanisms that limit the access of harmful substances to the organism, which is considered as the most effective barrier; these mechanisms are confirmed by GALT. The cellular components of GALT are in localized microenvironments, such as Peyer's patches (PPs), lamina propria, and mesenteric lymph nodes (MLN). The immune cells of GALT consist of T and B cells, macrophages and dendritic cells with distinctive phenotypic characteristics, and there are also other cell types within the system, including microfolded cells (M cells), Paneth cells, and intraepithelial lymphocytes. The hallmarks of the system are the sampling of luminal antigens by M cells and DCs, the antigen-driven priming and maturation of naive T and B cells, starting in PPs, their egress to MLN through lymphatics, and then their migration back to the LP of the gut, this is the homing of the immune cells in GALT1.
PPs are considered a major site for initiation of the local immune response in the gut via the capture of antigens by the lumen17-20. However; others consider the PPs as an amplification system. It has been also suggested that the site of priming mucosal B-cell response may occur in the lymph nodes21,22.
The present study describes the location and temporal appearance of the different subsets of B-cells in the GALT and the spleen, at different times in suckling and weaning rats. Furthermore, it shows that subpopulations of IgA B-cells and IgG B-cells appear early at 3 days old in MLN unlike PPs, where these subpopulations appear at 12 and 6 days, respectively, which may indicate that the immune response is triggered intestinal MLN originally.
Subjects and methods
Animals
Twenty-four suckling male Wistar rats were removed from the mother on 3, 6, 9, 12, 15, 18, 21, and 30 days of age. All animals were bred at UNAM vivarium where they were maintained at constant temperature and in light/dark cycles of 12 h each. Suckling rats were weaned on day 21 and fed with Purina Lab Rat Chow and water ad libitum. The suckling litters remained with the mother until the day of sacrifice. Two rat groups being formed for this purpose: group A, contained 3-, 6-, 9-, 12-, 15-, 18-, and 21-day-old rats, and group B, 30-day-old rats.
Tissues
Following anesthesia (Pentobarbital sodium, Sedalpharma), 1 cm of each tissue was sampled as follows: duodenum (DU), at 5 mm after cardias; jejunum (JE) samples were taken moving in caudal direction at 5 mm spacing for different rat's ages, but always taking as a reference 2 cm away from the stomach. The ileum (IL) was cut immediately at the cecal appendix (AP), PPs were taken from the distal JE; the whole cecal AP was excised; a caudal portion of spleen was taken; and all noticeable MLN were obtained.
All tissues were fixed with 4% paraformaldehyde in phosphate buffer 0.1M (pH 7.4) during 3 h at 4°C, and then kept overnight in 10% PBS-sucrose at 4°C. The tissues were embedded in OCT resin (Ames) and frozen at −70°C for further analysis.
Immunohistochemistry
For histological analysis, 5 µ slices of the different tissues were mounted on glass slides covered with poly-L-Lysine (Sigma Chemical Co., St. Louis, MO, USA). Conventional immunohistochemical procedures were performed23. Tissues were inhibited for their endogenous peroxidase activity with 3% methanol/H202, rinsed with 0.5% PBS-BSA, and incubated in monoclonal mouse biotin-conjugated anti-rat IgA (1:100), anti-rat IgG (1:100), and anti-rat IgM (1:100) antibodies (Serotec, Ltd., Oxford, England) for 1 h at 37° C. After rinsing with 0.5% PBS-BSA, sections were incubated for 30 min at 37°C with streptavidin-peroxidase complex (ICN Pharmaceuticals, Inc., Costa Mesa CA., USA). The slides were rinsed and the peroxidase reaction was developed with 3amino-9ethyl carbazole (Sigma Chemical Co., St. Louis, MO, USA) in acetate buffer 0.05M pH 5.5. Samples were counterstained with Mayer's hematoxylin and mounted using ace-mounting (Zymed Laboratories, So. San Francisco, Calif., USA).
Quantitative evaluation of positive cell
Positive cells were counted using an ocular grid, which was calibrated using a micrometer with a ×40 lens. Ten fields were randomly chosen for each tissue. The fields had a surface of 462,250 µm2; the results were converted to number of cells/mm2.
Statistical analysis
Differences between counts in areas and sections of tissues were examined using SPSS for Windows 20: The statistical analysis was performed by the Mann-Whitney U-test a non-parametric test with two rat groups being formed for this purpose: group A contained 3-, 6-, 9-, 12-, 15-, 18-, and 21-day-old rats, and group B 30-day-old rats.
Results
Intestine
B-cell subsets were mostly observed in the LP of the intestinal villus and crypts, beginning the surface expression of B-cells receptor on day 3. B-cell subsets increased with age, reaching the maximum number at weaning (Figs. 1-3).

Figure 1 Number of IgA B-cells in the duodenum, jejunum, ileum, ileocecal appendix, Peyer's patches, lymphoid nodes, and spleen in rats of 3, 6, 9, 12, 15, 18, 21, and 30 days old, with immunoperoxidase technique. Each point corresponds to three rats ± E-std.
IgA-positive B-cells were absent in all portions of the small intestine in 3-day-old rats, but they began to appear by day 6 when a few scattered cells surrounding the intestinal crypts were observed in the LP. B-cells bearing IgA receptors increased with age (Fig. 1) reaching the highest number at weaning (Figs. 1 and 4A and B). There were significant differences in the number of IgA-positive B-cells between Group A and Group B (DU p < 0.05, JE p< 0.001, and ileum p< 0.001).
On day 3, IgM-positive B-cells were observed in the small intestine. These IgM-positive B-cells were observed in DU, JE, and ileum (IL); and they increased with age (Fig. 3). Significant differences were only found in the number of IgM positive B-cells in the IL (p < 0.05). Enterocytes were negative to anti-IgM antibodies at all ages.
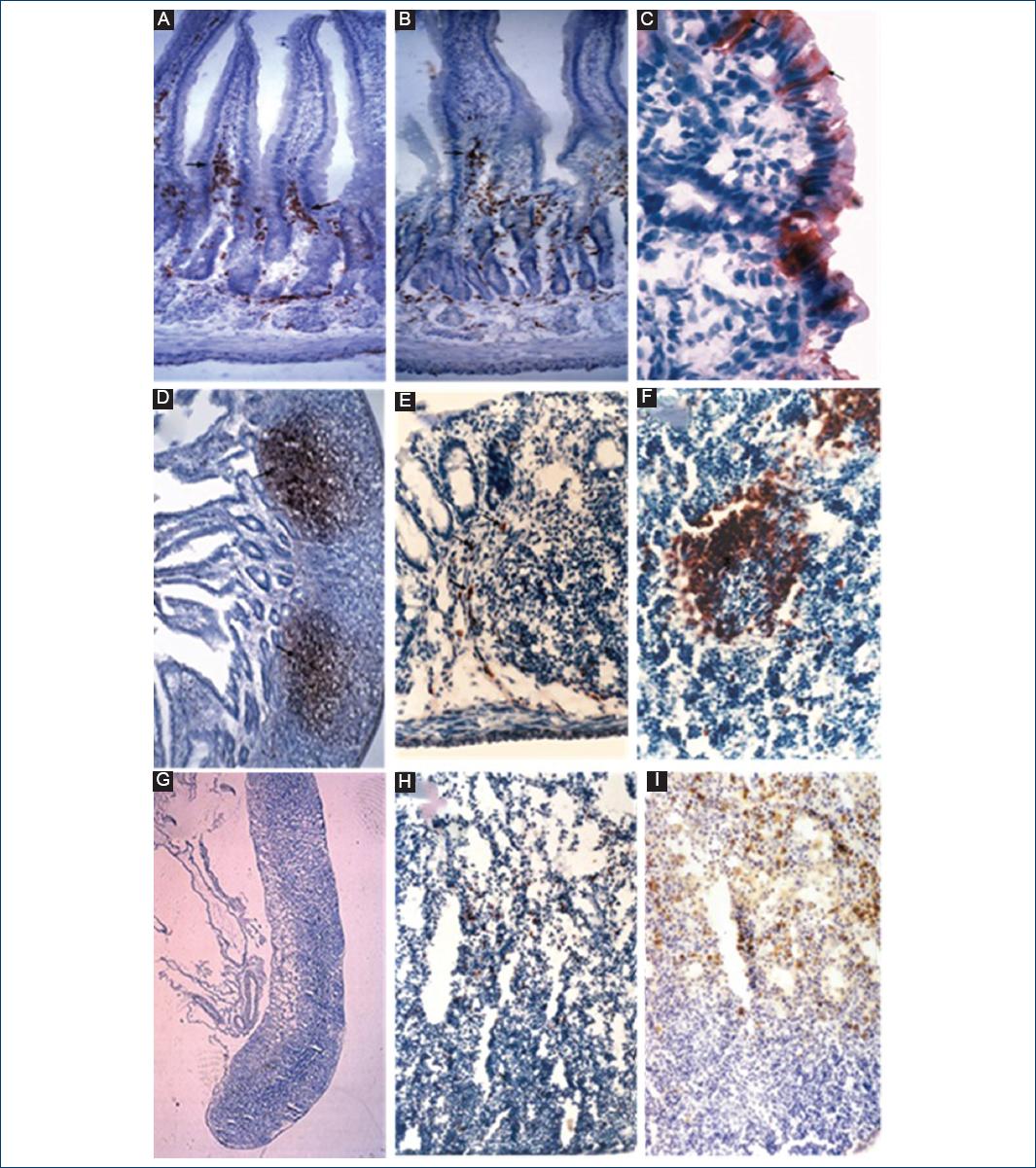
Figure 2 Distribution of positive immunodetected B cells in the gut (GALT) system and spleen of suckling rats. A and B: IgA B-cells (arrows) in lamina propria of the duodenum and ileum (×20); C: IgA positive appendix (AP) enterocytes (×100); D: IgM B cells in the lymphoid follicle (LF) of Peyer's patches (×20); E: IgM B cells in the LF of ileocecal AP (×40); F: IgM B cells in the secondary LF of spleen (×40); G: lymph node (×4); H: IgM B cells in the lymph node (×40); I: IgA B cells in the lymph node (×20). Stained with biotin-conjugated monoclonal mouse anti-rat antibodies.
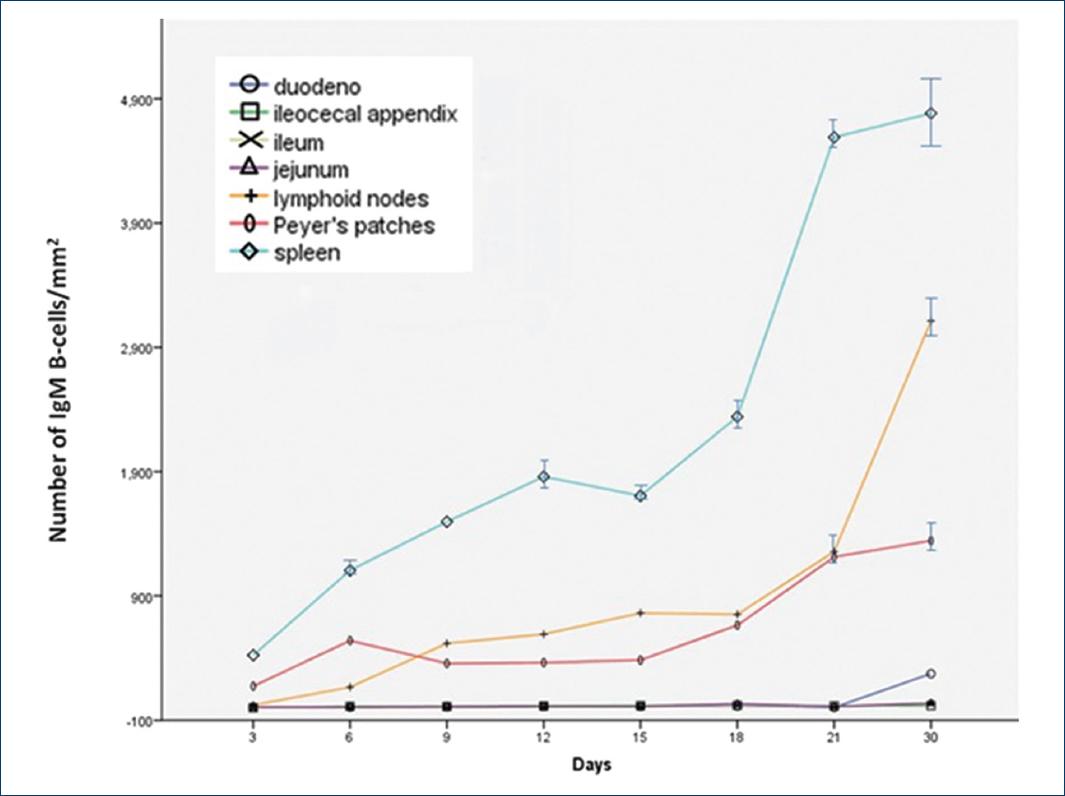
Figure 3 Number of IgG B-cells in cells in the duodenum, jejunum, ileum, ileocecal appendix, Peyer's patches, lymphoid nodes, and spleen in rats of 3, 6, 9, 12, 15, 18, 21, and 30 days old, with immunoperoxidase technique. Each point corresponds to three rats ± E-std.
The anti-IgG antibody gave diffuse staining in the intestine LP. There were no IgG-positive B-cells on day 3; however, a few cells were seen on day 9, which became clear at weaning when the maximum level was reached (Fig. 2). Significant differences were only seen in the number of IgG-positive B-cells for the JE (p = 0.005) and the ileum (p < 0.005).
It was evident that all subsets increased at weaning. This increase was more pronounced in subpopulations of IgA-positive B-cells and IgG-positive B-cells.
On day 3, IgA-positive enterocytes (Fig. 4C) were present in the JE and ileum, but none were found in the DU. By day 9 positive enterocyte staining disappeared.
Cecal AP
Rat AP grew fast, and by the 9th day, it was larger than the stomach. On day 3, the AP showed no subset of B-cells. IgM-positive or IgG-positive B-cells appeared on day 6 and were mostly located in the marginal zone of the primary lymphoid follicle (LF) (Fig. 4E); IgA-positive cells were present on day 9. In the AP, all B-cell subsets increased with age (Figs. 1-3). In addition, in a similar way to the intestine, cecal AP enterocytes were positive to IgA from day 3-6 (Fig. 4C). There were no significant differences between the subsets of B-cells in the AP.
PP
B-cells of PPs showed all subsets of B-lymphocytes. They were present in the lamina propria (LP) of the intestinal villi in the sub-epithelial region known as the dome area. However, IgA-positive B-cells were observed surrounding PP in the interfollicular area until day 12 and on day 30; the number of this cell subset increased significantly (Fig. 1). The statistical analysis showed that there was a significant difference (p < 0.005) in the number of IgA-positive B-cells between the rats in Group A and those in Group B.
IgM-positive cells were present in the PP by day 3 (Fig. 4D) and IgG- positive cells at day 6 with few cells being scattered in the periphery. These cell subsets increased progressively, but at weaning IgG subsets increased significantly (Figs. 2, and 3). There were significant differences between the number of IgM-positive B-cells (p < 0.001) and IgG-positive B-cells (p < 0.05) between Group A and Group B.
MLN
In the newborn rat, the MLNs were long in shape (Fig. 4G and H) and were clearly divided into two zones: a dorsal zone densely populated with diffuse lymphoid tissue and lymph follicles; and a ventral zone poorly populated with lymphocytes, which had apparent reticular fibers and numerous macrophages located in the medullary cords (Fig. 4H). At 30 days of age, the shape of MLN looked like that of an adult rat; the dorsal zone corresponds to the cortex and the ventral zone to the medulla of the lymph node (Fig. 4H and I).
All B-cell subsets were present from 3 days of age and increased progressively. IgA-positive B-cells were mainly scattered throughout the dorsal zone in the diffuse lymphoid tissue. There were significant differences in the number of IgA-positive B-cells between Groups A and B (p < 0.001). Numerous IgM-positive and IgG-positive B-cells were mainly located in the MLN, although some could be observed in the diffuse tissue as well, and there were significant differences (p < 0.001) for both subsets of B-cells.
At all suckling ages, there were more IgM-positive B-cells than IgG-positive B-cells, and at weaning both subsets of B cells were increased (Figs. 2 and 3).
Spleen
In neonatal rats, the spleen contained large areas of hemopoietic red pulp and only small zones of white pulp, which were represented by accumulations of lymphocytes around the arterioles. In general terms, immunoperoxidase staining demonstrated that B-cells were distributed in both white and red pulp, and all B-cell subsets increased with age (Figs. 1-3). The statistical analysis only showed significant differences in the number of IgG-positive B-cells (p < 0.05) and IgM-positive B-cells (p < 0.005) between Group A and Group B.
IgA-positive B-cells appeared on day 6 forming clusters in the periarteriolar lymphoid sheath (PALS).
IgM-positive B-cells were observed to be scattered in the red and white pulp of the rat's spleen for 3 days of age. However, on day 6, these IgM-positive B-cells were located mostly in the white pulp: in the PALS and mainly in the manto and dark zone of the LF germinal centers (Fig. 4F). IgG-positive B-cells had a similar pattern both in appearance and distribution as IgM-positive B-cells. In all ages of suckling rats, IgM-positive B-cells were more numerous than IgG-positive B-cells, but at weaning, IgG-positive B-cells increased, but not to the level of the IgM-positive B cells (Figs. 2 and 3).
Megakaryocytes, located in red and white pulp, were observed in rats for 3 days of age. These cells were stained with anti-IgG antibodies, but not with anti-IgM and anti-IgA antibodies. The number of megakaryocytes was reduced at weaning.
Discussion
It is known that different factors modulate immune responses, but in the digestive tract, breast milk may play an important role in the maturation of the immune response, mainly in relation to GALT5. In this sense, it has been demonstrated that besides nutrients and antibodies, breast milk contains cells6,7, hormones, cytokines, and growth factors8-11. All of these biologically active factors not only facilitate the development of essential digestive functions but also regulate the maturation of the intestinal mucosal barrier12. Colostral cells are immunologically reactive and elicit cell-mediated immunity and synthesize immunoglobulins, predominantly those of the IgA class13.
On the other hand, the adaptive coevolution of mammals and bacteria led to the establishment of commensal and symbiotic relationships that have contributed to the development of the immune system and maintenance of normal physiology24,25. Indeed, recognition of commensal bacterial products through toll-like receptors plays a critical role in epithelial homeostasis, by inducing secretion of protective factors which strengthen epithelial resistance to pathogens and plays an important role in the induction of the immune response in the intestine26.
In the present study, all subsets of B-cells increased with age; this is largely due to the different factors present in breast milk. IgM B-cell subsets were the first to colonize the GALT and spleen. B-cell subsets are modified during cell development to be activated. Immature B-cells that have never been exposed to an antigen are known as naive B-cells and express IgM or IgD molecules on the cell surface. Activation of B-cells induces immunoglobulin class switching, which results in the transformation of B-cells into plasmatic cells that produce different antibody isotypes. In this work, this is supported by the posterior appearance of IgA and IgG B-cell subsets in the GALT and spleen. Many factors may induce the switching of IgM-positive B-cells to IgA-positive and IgG-positive B cells: the special microenvironment generated by lymphoid and non-lymphoid elements such as enterocytes, fibroblasts, follicular dendritic cells, Il-6, Il-10, and probably other cytokines and chemokines produced by LP stromal cells27,28, besides external factors such as microorganisms and breast milk. In this sense, it is well known that breast milk contains growth factors, which can play a role in the maturation of GALT8,9. Nucleotides from milk have a determinant action in the development of the GALT8. In addition, a proline-rich protein10 and breast milk L-glutamine29 participate in the proliferation of lymphoid cells in TGI, while EGF not only enhances proliferation and differentiation of epithelial cells but also has significant effects on healing damaged mucosa or intestinal injury adaptation11.
The observations from this study indicate that the number of all B-cell subsets in the gut increases with age; but at weaning, when higher ingestion of microorganisms occurs, there is an important increment of IgA and IgG-positive B-cells as well as a stabilization of the IgM subset. It is likely that bacterial lipopolysaccharides (LPS) induce switching of B-cells from IgM to B-cells IgG3 and IgG2b, even in the absence of T-cells as described by Rothman30. Furthermore, transforming growth factor β enhances secretion of IgA only when IgM-positive B-cells are stimulated by LPS31.
PPs are considered a major site for initiation of the local immune response in the gut through the capture of antigens by the lumen17-20. However, others consider the PP as an amplification system. It has been also suggested that the site of priming mucosal B-cell response may occur in the draining lymph nodes21,22. Van den Broeck et al.32 used F4 fimbriae of enterotoxigenic Escherichia coli to induce stimulation of the mucosal immune system in pigs, and the results showed that antibody-secreting cells of the IgG and IgA isotypes appear in MLN on the 7th day of age, and in PPs on the 11th day of age. These data support the fact that antigens initially interact with MLN cells and subsequently with PPs cells amplifying the system32.
The observations in this study showed that all B-cell subsets appear in the MLN on the 3rd day of age. However, IgA and IgG-positive cells were absent at this time in the LP, AP, and PPs. The largest number of IgA-positive and IgG-positive B-cells was observed in MLN, in 3-30-days-old rats. The most significant changes in B-cell subsets were in the MLN. It is possible that the antigens that reach the intestine are captured by intestinal lymphatic vessels and then transported via afferent lymphatic vessels to the MLN. In the MLN, these antigens may have induced B-cell differentiation from IgM B-cells to IgA-positive and IgG-positive B-cells, which migrated to the secretory and systemic immune system. Furthermore, migration of lymphocytes from the MLN to the LP and PPs may occur because they share specific vascular addressins in the high endothelial venules33,34.
The staining of ileon and AP enterocytes by IgA on day 3 could be due to endocytosis of colostral IgA. It has been demonstrated that colostrum IgG molecules are endocytized through ligand-receptors, predominantly in the distal portions of the rat's small intestine35,36. These facts may explain the presence of IgA in the cytoplasm of newborn rat enterocytes. On the other hand, it is considered that the IgA mediates intracellular neutralization of endotoxins within epithelial cells and thus inhibits the proinflammatory pathway normally stimulated by endotoxins37.
In the suckling rat's spleen, all subsets of B-cells were detected. On the 3rd day of age, IgM-positive B-cells were the most abundant subsets, and the IgM-B cells were scattered in the white pulp and around the vessels. These findings concur with those of Dijkstra38, who described the appearance of B-cells in the spleen at birth. At weaning, IgG-positive B-cells increased significantly. This may be due to the antigenic stimulation by micro-organisms, which induces switching from IgM to IgG B-cells30.
In general terms, the present findings show a progressive increase of B-cells in all the tissues studied, including a sudden increment at weaning. It has been published that breast milk contains antibodies, immunologically reactive cells6, mitogenic factors10,29, cytokines, and growth factors8. We think that probably these factors are the inducers of the growth and differentiation of immunological cells and may have an important role in the development of GALT. The present study about normal development and distribution of B-cell subsets in different organs of the GALT and the spleen contributes to the knowledge of the effects of suckling and weaning on tissues studied in the rat model. Furthermore, this study shows the presence of all B cell subpopulations studied in MLN at 3 days of age unlike what happens in PPs, where activated B cell subpopulations appeared at 6 and 12 days old. This allows us to propose that priming originally occurs in MLN and later in PPs.











 nova página do texto(beta)
nova página do texto(beta)



