Introduction
Changes in society, technology, and microorganisms alone contribute to the emergence of new diseases, to the re-emergence of already controlled diseases, as well as to the development of antimicrobial resistance. At present, pathogens resistant to several antimicrobials have proliferated, both in humans and in veterinary medicine.
Gastrointestinal diseases in infants are one of the public health problems worldwide, especially in developing countries such as Mexico.
In 2015, the World Health Organization estimated that diarrheal diseases cause more than half of the global burden of foodborne diseases, with 550 million people sick and 230,000 people dying every year. Diarrhea is usually due to the ingestion of poorly cooked foods or dairy products contaminated with norovirus, Campylobacter, nontyphoidal Salmonella, and pathogenic Escherichia coli1. In Mexico, intestinal infections represented the second cause of morbidity, with more than five million cases in 2011, which placed this condition as an important public health problem, since the national incidence increased for the third consecutive year, with the most vulnerable sector being children under 5 years of age2.
In 2013, acute diarrheal disease (ADD) was the second cause of morbidity in the population under 5 years of age in the state of San Luis Potosi (S.L.P.), where the disease can present serious complications in children, due to some circumstances such as having a birth weight of < 2.5 kilos, have not received breastfeeding, have some degree of malnutrition, and do not have the complete vaccination schedule according to their age3. In 2013, the number of children under 5 years with ADD was approximately 40 thousand cases4. The incidence of ADD in this state may be due to the social backwardness, since according to the National Council for the Evaluation of Social Development Policy (CONEVAL) in the year 2012, SLP occupied tenth place in social backwardness at the national level, while Mexico City was located at 31 places, with a very low degree of social lag5.
Among the virulence factors of E. coli are adhesins and toxins. Pathogenic strains of E. coli have specific adhesion factors6, which allow them to colonize the intestine. These adhesions can be divided into two groups: fimbrial adhesins and afimbrial adhesins7. The afimbrial adhesins are outer membrane proteins such as intimin6.
Bacterial toxins are of two types, lipopolysaccharide endotoxins and exotoxins. Exotoxins are classified as enterotoxins, cytotoxins, and neurotoxins. Enterotoxins produce watery diarrhea and inflammatory diarrhea cytotoxins, whose bowel movements contain blood and mucus7. Once E. coli has colonized the intestine, the damage is caused by the production of enterotoxins, cytotoxins as well as by invasion of the target cell.
Based on their mechanism of damage, E. coli are classified into six pathotypes: Enterotoxigenic (ETEC), Enterohemorrhagic, also known as producers of STx toxin (EHEC), Enteropathogenic E. coli (EPEC) is classified in tEPEC (they have the bfpA + genes) eaeA) and aEPEC (possesses the eaeA gene), Enteroaggregative E. coli (EAEC), enteroinvasive (EIEC), and diffuse adherence E. coli6.
Savarino et al.8 have considered the astA gene, which codes for the EAST1 enterotoxin, as a characteristic of the EAEC group. On the other hand, Nataro et al.9 recognized two different phenotypes of E. coli, belonging to the enteroadherent strain: diffuse and enteroaggregative, this discovery is the first description of EAEC. The enteroaggregative adhesion is characterized by a formation of "stacked brick" (stacked-brick in English), where bacteria adhere to both the cell surface and the glass of the preparation. The reproducibility presented by this trial has made it a standard test for the determination of the aggregative adhesion phenotype and thus the identification of the EAEC virotype. In addition to, molecular assays such as the production of the EAST1 toxin, encoded by the astA gene8, the aat gene, which codes for a protein called dispersion, as well as the chromosomal gene AggR present in a pathogenicity island of E. coli was identified10.
In nature, microorganisms rarely live in isolated colonies of a single species, generally, living in communities called biofilms11. Biofilm is a term used to define biological systems or a matrix with bacterial populations adhered to each other and/or to a surface. This structure allows bacteria to take refuge from harmful factors such as the environment, antibiotics, and immune system, among others11.
The architecture is not solid, on the inside microcolonies are communicated by microchannels that favor the entry of nutrients and elimination of waste products12.
Recently, it was found that the moment when E. coli begins to form the biofilm, the bacteria become ovoid and smaller to eventually enter a stationary phase where they stop dividing. As this occurs, they begin to produce and excrete autoaggregation curli fibers. These curli fibers, at the molecular level, are proteins assembled in an amyloid structure, which allows their staining with Congo Red13.
The presence of biofilm produced by E. coli had been considered a very important virulence factor since it has been related to antimicrobial resistance. The formation of this structure was detected in catheters, medical instruments and heart valves, favoring the chronicity of the ailments and making their elimination difficult13.
For the study of the biofilm production of different microorganisms, several methodologies have been developed, among them are the Christensen Tube Method and the Congo Red in agar plates. Christensen et al.14 were recognized one of the first groups to develop methodologies to determine the production of biofilm. When conducting various investigations of intrahospital infections, they found a certain mucoid substance in catheters, heart valves, and prostheses, among others and were associated with infections caused by coagulase-negative staphylococci (usually Staphylococcus epidermidis) that produce what they called at that time, slime.
Ensuring that the Christensen method is not always successful in determining biofilm production and that variations in the culture medium affect the results, Freeman et al.15 described an alternative methodology to detect the production of biofilm on a solid medium. This method is based on the coloration of the bacterial colonies, thanks to the Congo red dye present in the brain-heart infusion agar plates.
In both Latin America and North America, epidemiological studies were conducted and the purpose was to determine different pathotypes associated with diarrhea cases in children. These studies demonstrated that E. coli that belongs to the ETEC and EPEC pathotypes are the main isolated pathogens15-17. In regard to Mexico, since 1987, several studies showed the presence of EPEC in most of the isolates obtained from patients with a prevalence of 17-19% of cases of infant diarrhea18. In 2005, Estrada et al. reported the presence of four E. coli pathotypes in a community bordering Mexico City.
On the other hand, despite the efforts made for the prevention and control of infectious diseases, they continue to represent a global public health problem due to both emerging and re-emerging microorganisms.
Among the pathogens that are emerging and resistant to antimicrobials are E. coli, which affects humans and different animal species. Multiple investigations in both humans and animals show resistance to the most frequently used antimicrobials19-22. Bacteria are generally resistant to more than one antimicrobial21. Studies in Mexican children show that these bacteria cause diarrhea disease in hospitalized children and are resistant to trimethoprim-sulfamethoxazole (TMP-SMX) and ampicillin (AM). These drugs are mostly used in the treatment of pediatric diarrhea17. In the State of S.L.P., there are no studies in this regard.
For the aforementioned, the objective of the present work was to compare the E. coli isolated from children with diarrhea obtained from two states with different degree of social lag and establish the virotypes, adhesion to HEp-2 cells, biofilm formation and antimicrobial resistance of said bacteria.
Materials and methods
Bacterial strains and characterization
The study was conducted from 38 stool samples from children with diarrhea from the Dr. Ignacio Morones Prieto Central Hospital in the state of SLP and 27 stool samples from children from the National Institute of Pediatrics (INP) of Mexico City. The samples were taken by highly qualified personnel; later they were transported to the laboratory in the Cary Blair medium. Once in the laboratory, they were planted on MacConkey agar medium (DIBICO®) and incubated at 37°C for 24 h. One to five colonies were selected, with morphological characteristics of E. coli. Subsequently, conventional biotyping tests were performed to confirm the presence of E. coli. From these tests, 100 strains of E. coli from S.L.P. and 100 strains of E. coli from Mexico City were obtained. In addition to these biochemical tests, the production of b hemolysis was evaluated. To do this, the strains of E. coli were seeded on 5% sheep blood agar, incubated at 37°C for 24 h and subsequently the b hemolysis was evaluated by observing the total destruction of the erythrocytes. Reference strains A panel of E. coli reference strains were used as positive controls for the different polymerase chain reaction (PCR) assays, antibiograms, and adhesion to HEp-2 cells. Strains TB334C (Stx1 +, Stx2 +, eaeA +), B-171 (BfpA +, eaeA +), EIEC O124: H30 (ial +), and O407: H1 (LT +, ST +) were kindly donated by Dr. Estrada García of the Research Center and Advanced Studies (CINVESTAV), EAEC 044: H18 (aat, astA) strain donated by Dr. Chattaway of Public Health England. Finally, O42 and C600 (K12) strains were provided by the National Epidemiological Reference Institute of Mexico (INDRE).
DNA extraction and PCR reaction
The genomic DNA of the E. coli isolates was extracted by the boiling method, previously described by Al-Gallas et al.23. The PCR (single or multiple) allowed to detect genes that code for the toxins (LT, STa, Stx1, Stx2, and EAST1) and genes that code for adhesins (intimina, eae, pili Type IV, bfpA, for invasiveness and gene that codes for dispersin, aat). The PCR reactions used for the amplification of the different genes were carried out using Apex Taq DNA polymerase (Genesee Scientific, USA) and primers. The 50 µl reaction mixtures contained the following: 1X buffer (100 mM Tris-HCl, pH 8.5, 500 mM KCl, 15 mM MgCl2, 1% Triton X-100), 0.2 mM of each dNTP (Apex dNTP mixture, 10 mM), 0.25 µM of each primer, 2.0 U of Apex Taq DNA polymerase, 3 µl of tempered DNA, and 37 µl of nuclease-free water. The multiplex PCR products (mPCR) were separated on a 2% Agarose gel (Amresco, Ohio, USA). The amplified PCR products were visualized with ethidium bromide under a UV illuminator and recorded using the Digidoc Imaging System (UVP, CA, U.S.A.).
Antimicrobial susceptibility assays
The antimicrobial susceptibility test was carried out to determine the profiles of susceptibility and resistance of the bacteria to the antibiotics tested. For this, the paper disc diffusion method was used. Impregnated antimicrobial discs with AM (10 mu.g), amikacin (30 µg), kanamycin (30 µg), cephalothin (30 µg), amoxicillin/clavulanate (30 µg), cefotaxime (30 µg), TMP/SMX (1.25 mu.g/23.75 µg), and cefoxitin (30/mg). These discs (6 µm diameter) were placed in an automatic Sensi Disc dispenser (DB BBL Sensi Disc Antimicrobial Susceptibility Test Discs, Sparks MD, USA). The inoculated agar plates were incubated at 37°C for 18-24 h. After incubation, the diameters of the zone of inhibition of the antibiotic were measured. The results were used to classify the isolates as resistant, intermediate, or susceptible to a particular antibiotic, using standard reference values as shown in the National Committee for Clinical Laboratory Standards 2014. Multidrug resistance defined as resistance to three or more different classes of antibiotics.
Adhesion test in HEp-2 cells
One hundred strains of E. coli previously identified as EAEC (ast1 + and/or aat +) were tested for adhesion to HEp-2 cells, as described by Donnenberg and Nataro24, with modifications. In summary, 6.0 × 10 5 HEp-2 cells were cultured in DMEM medium with high glucose content (Biowest, Miami FL, USA) supplemented with 10% fetal bovine serum (Biowest, Miami FL, USA) and 1% antibiotic/antifungal solution (PAA, Pasching, Austria). The cells were placed in 24-well plates (TPP, Switzerland) containing a glass lentil and incubated until reaching a confluence of 80-90% (1-2 days). The HEp-2 cells were washed 3 times with sterile PBS, 500 µl of fresh DMEM medium without fetal bovine serum or antibiotics were added.
One day before the experiment, the E. coli strains were grown overnight in 1.5 ml of LB medium with 1% D-mannose and the concentration was adjusted to 0.5 UMF (2 × 108 CFU/ml) with sterile PBS. The cultures were centrifuged and then resuspended in 1 ml of sterile PBS. 50 µl of the bacterial suspension was mixed with 450 µl of DMEM medium without fetal bovine serum or antibiotics and inoculated into cultures of HEp-2 cells. The infected cells were incubated for 3 h at 37°C under a 5% CO2 atmosphere and washed 5 times with sterile PBS, fixed with absolute methanol for 1 min and stained with 10% Giemsa. Adherence patterns were assigned according to the description of Nataro and Kaper6.
Controls in each experiment included strains of E. coli O42 that showed aggregate adhesion and E. coli K12 that did not bind to HEp-2 cells.
Results
The present study was carried out with 200 strains of E. coli, 100 of them from the Central Hospital Dr. Ignacio Morones Prieto of the state of S.L.P., and 100 from the INP, from the Mexico city.
The b-hemolysis assay showed that of the 200 strains of E. coli studied, 25 had b-hemolysis, of which 14 were from Mexico city and 11 from S.L.P.
Identification of virulence genes of E. coli
Through the PCR assay, nine virulence genes were identified, of which five code for toxins (LT, ST, STX1, STX2, and astA), two for adhesins (bfpA and eaeA), one for invasiveness (ial), and a gene that participates in the secretion of the protein called dispersin, aat.
As can be seen in Table 1, of the strains of E. coli isolated from the Mexico city, 30% presented the LT gene; 14% the ST gene, 14% the astA gene, and 4% the ial gene. While for the state of S.L.P., 57% of the strains presented the LT gene; 37% presented TS; 27% presented the LT: ST genes; 28% presented the ial gene, 22% presented the STX1 gene, 13% the antler gene, and 9% presented the eaeA gene. These being the most prevalent among the nine genes studied. The statistical analysis showed significant differences (p < 0.05) for the LT, ST, LT: ST, ial, and STX1 genes.
Table 1 Percentage of genes found in E. coli strains from each population studied
| 1Genes | % of Strains Mexico City | % of Strains S.L.P. |
|---|---|---|
| LT* | 30 | 57 |
| ST* | 14 | 37 |
| ST: LT* | 2 | 27 |
| ial* | 4 | 28 |
| St × 1* | 3 | 22 |
| St × 2 | 0 | 9 |
| St × 1:St × 2 | 0 | 5 |
| St × 1:St × 2:eaeA | 4 | 0 |
| eaeA | 9 | 9 |
| bfpA | 2 | 0 |
| astA | 14 | 13 |
| aat | 3 | 1 |
| astA:aat | 3 | 0 |
Genes that code for: LT: heat-labile toxin; ST: heat-stable toxin; St × 1 and St × 2: "Shiga-like" cytotoxins; eaeA: gene that codes for intimina; bfpA: codifies for pili hair formers;ial: invasiveness; astA: enteroaggregative toxin heat stable EAST1; aat: gene that participates in the secretion of the protein called dispersin.
*p < 0.05; S.L.P.: San Luis Potosí.
Virotypes
The identification of virulence genes allowed us to classify E. coli into virotypes. This classification was made according to what was published by Estrada-García et al.17 (Fig. 1) and obtained the following results. For Mexico City: ETEC was 49% and in SLP was 67%. The statistical analysis showed a significant difference with a p = 0.009. EAEC in Mexico City was presented in 14% and in S.L.P. was 14%. EIEC in Mexico City with 4% and in S.L.P. was 28%, and the statistical analysis showed a significant difference of p = 0.000. EHEC for Mexico City with 3% and in S.L.P. was 30%, and the statistical analysis showed a significant difference with a p = 0.000. The percentage of strains that were aEPEC in Mexico City was 9% and for S.L.P. was 5%.
Adherence assay to HEp-2 cells
Adherence is one of the first factors for pathogenic E. coli strains to cause diarrheal infections. For this purpose, a gold standard test was considered for the identification of the EAEC virotype. This test consists of adherence of the bacteria to a monolayer of HEp-2 cells.
As can be seen in table 2, of the 17 strains of E. coli that were evaluated for Mexico City, only one strain presented aggregative adherence (AA) and it was astA +. Twelfth strains showed diffuse adherence (AD) and of which two presented both astA and aat genes. The remaining four strains did not present any adherence pattern. Patterns of adherence of E. coli to HEp-2 cells (Fig. 2).Figure 2A shows negative control of adhesion of E. coli and we used the strain K-12. Figure 2B shows the positive control of aggregate adhesion and we used the strain O:42. Figures 2C, D, E and F, show different patterns of adherence, these strains of E. coli were isolated of samples from the CDMX and S.L.P.; C) shows diffuse adherence, the E. coli strain comes from S.L.P.; E) Shows aggregative adherence, the E. coli strain comes from the CDMX; F) shows aggregative adherence and the E. coli strain comes from S.L.P.
Table 2 Type of adherence and genes that carry strains of E. coli
| Location | Number of strains | Genes | Type of adherence | ||
|---|---|---|---|---|---|
| AA | AD | N | |||
| CDMX | 14 | astA+ | 1 | 10 | 3 |
| 0 | aat+ | 0 | 0 | 0 | |
| 3 | astA+:aat+ | 0 | 2 | 1 | |
| Total Strains | 17 | ||||
| S.L.P. | 13 | astA+ | 4 | 4 | 5 |
| 1 | aat+ | 0 | 0 | 1 | |
| 0 | astA+:aat+ | 0 | 0 | 0 | |
| Total strains | 14 | ||||
AA: aggregate adherence; AD: diffuse adhesion; N: negative adhesion; S.L.P.: San Luis Potosí.

Figure 2 A: Negative control Escherichia coli strain K:12 ×100; B: Positive control aggregate adhesion strain E. coli O:42 ×100; C: Diffuse adhesion strain ×100 Mexico City; D: Diffuse adhesion strain ×100 San Luis Potosi (S.L.P.); E: Aggregates adhesion strain ×100 Mexico City; F: Aggregates adherence strain ×100 S.L.P.
For S.L.P., 13 strains presented the astA gene of which four presented AA, four presented AD, and five strains did not present adherence. Regarding the strain that possesses the aat gene, it did not show adherence (Table 2).
Biofilm production
To evaluate the biofilm production, the Congo red and Christensen agar assays were used for which the strains that showed mobility were taken into account. Of the E. coli isolated from the CDMX, 60% were mobile and 84% for S.L.P.
Congo red agar assay
Of the 60 strains of flagellated E. coli isolated from the CDMX, only 42 strains formed biofilm and of which 11 were strong producers of biofilm (++), 31 were considered weak producers of the biofilm (+), and 18 strains were negative. For SLP, of the 84 strains of flagellated E. coli, only 64 strains formed biofilm, of these 28 were strong producers (++), and 36 strains were weak producers of biofilm (+), and 20 of them were negative (Table 3). In figure 3, the staining acquired by the colonies when producing biofilm is observed.
Table 3 Biofilm evaluation of E. coli strains by the Congo red agar method (ARC)
| Red agar | Congo | |
|---|---|---|
| CDMX n (%) | S.L.P. n (%) | |
| (++)1 | 11 (19) | 28 (33) |
| (+)1 | 31 (51) | 36 (43) |
| Total positive strains | 42 (70) | 64 (76) |
| (-)1 Total negative strains | 18 (30) | 20 (24) |
| Total strains evaluated | 60 (100) | 84 (100) |
1(++) strong biofilm-producing strains, (+) weak biofilm-producing strains.
(−) non-biofilm-producing strains. S.L.P.: San Luis Potosí.
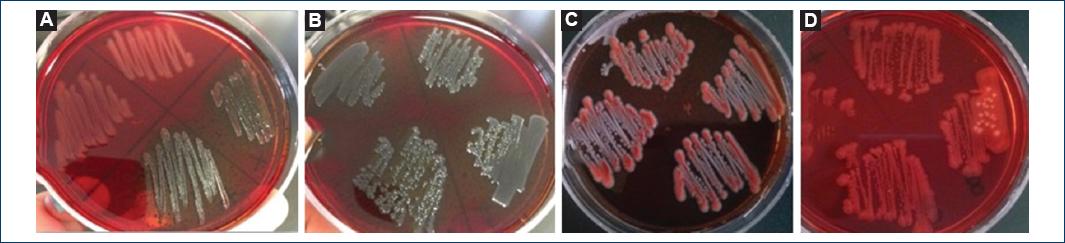
Figure 3 A: Upper strain O:42 negative control (colorless colonies), lower part K strain: 12 positive control (crystalline dry black colonies); B: (++) dry black crystalline colonies; C: Pink colonies with the black center (+); D: Colorless colonies, negative control; (B-D) Belong to strains of San Luis Potosi.
Christensen method (tube method)
According to the Christensen method, of 60 flagellate strains of E. coli for CDMX, 11 strains were biofilm producers, while for S.L.P. of 84 flagellate strains of E. coli, 18 strains were biofilm producers. The number of strains that were not biofilm producers for CDMX was 48 strains and 66 strains for S.L.P. (Table 3).Figure 4 shows the staining of the tube wall inoculated with a biofilm-producing strain.
Antibiogram
To evaluate the resistance and sensitivity to certain antibiotics, antibiogram tests were performed. As we can see in figure 5, the strains of E. coli isolated from Mexico City showed 75% resistance to AM, while the strains from SLP showed 51% and the statistical analysis showed a statistically significant difference with p = 0.000. About 53% of the strains of Mexico City were resistant to cefalotin (CF) and in S.L.P., only 31% with p = 0.001. About 52% of the Mexico City strains showed resistance to SMX/TMP in relation to 37% of strains of S.L.P. with p = 0.03. 41% of the strains of Mexico City showed resistance to amoxicillin/Clavulanic acid in relation to 13% of strains of S.L.P. with a statistically significant difference of p = 0.000. About 33% of the strains were resistant to kanamycin in Mexico City, in relation to 3% of the strains of SLP and the statistical analysis showed p = 0.000. 33% strains in the CDMX and 3% in SLP presented resistance to cefotaxime, with p = 0.000.
Discussion
The bacterium E. coli is the etiological agent of diseases of the digestive tract, urinary tract, and central nervous system. At present, it is known that there are several factors related to genotypic changes of this bacterium, from which derives the importance of determining not only the bacterium as the causative agent of diarrhea but also to identify the virulence genes related to the disease.
Although there are reports in which the prevalence of different pathotypes was determined, these studies come from populations from different geographical areas, different development conditions and different age groups, which makes it difficult to carry out comparative studies.
In the present work, to compare the E. coli virotypes from two states of the Mexican Republic and evaluate both genotype and phenotype, stool samples were obtained from children with diarrhea from SLP and Mexico City. The population are different both in geographical characteristics and in their population composition. According to data obtained from the National Institute of Statistics and Geography (INEGI), 36% of the population in SLP and only 0.5% in Mexico City are considered rural and with differences in the degree of social lag.
The results showed a higher incidence of E. coli virotypes isolated from stool samples from S.L.P. in relation to the virotypes of this bacterium identified from Mexico City.
The virotype that presented a higher prevalence in both study groups was ETEC. In Mexico City 49% and S.L.P. About 67% of the strains and the statistical analysis showed a significant difference (p = 0.009). The data obtained in this work agree with that published by Nataro and Kaper6 Paniagua et al.3 and Qadri et al.25 who affirm that ETEC is the most frequently found pathotype in developing countries as well as in developed countries, and the high prevalence of ETEC is related to its capacity for colonization6.
Regarding the virulence genes identified for ETEC, the genes that code for LT and ST toxins were those that occurred most frequently in the strains studied. The prevalent gene for both groups was the one that codes for the LT toxin. The strains isolated from Mexico City presented 30% and SLP 57% of the strains. This is concordant with Cortes et al.26, Jiang et al.27, Paniagua et al.3, Estrada-García et al.28, and Qadri et al.25.
Given that, E. coli strains were from fecal samples taken during the spring and summer seasons, it is likely that the incidence of the ETEC pathotype is seasonal, and this is in line with that published by Estrada-García et al.28.
The EHEC virotype was the second most frequent in the state of S.L.P., with 30% of the positive strains and in the CDMX with 3%. The statistical analysis showed a significant difference (p = 0.000). The high prevalence of this virotype in the S.L.P. population is striking compared to both Mexico City and that published by other authors. Estrada-García et al.28 showed that the frequency of EHEC in Mexico City was 2.5%. Vilchez et al.29 in a study conducted in Nicaragua reported 2.1%, Nataro and Kaper6 mentioned that this virotype is isolated less frequently in the underdeveloped population compared to the ETEC and EPEC virotypes. However, according to Clarke et al.30, EHEC has become an important virotype due to the increase in the number of outbreaks that have appeared in both developed and developing countries and mentions that this increase may be due to the high consumption of badly cooked food.
Despite the high prevalence of EHEC in S.L.P., there is no report from the health authorities of the state regarding the outbreaks of hemolytic uremic syndrome, as occurred in Germany in the year 201131.
Like EHEC, the prevalence of the EIEC virotype was higher in the state of S.L.P. (28%) and for Mexico City (4%) finding a statistically significant difference (p = 0.000). Estrada García et al.28 found 5% of this virotype, which coincides with our data in Mexico City and differs with the results obtained in S.L.P.
Clarke et al.30 as well as Escher et al.32 considered the EIEC virotype as the causal agent of morbidity and mortality in both children from developed and developing countries due to poor sanitary and hygiene conditions. It is likely that the high prevalence of this virotype in S.L.P. is related to that published by Clarke et al.30 given that S.L.P. presents a high degree of social lag.
The EPEC virotype was presented in 9% of Mexico City and 5% of SLP strains. Paniagua, et al3., in a study in children with diarrhea in Mexico City determined the presence of EPEC with a percentage of 9.3% similar to that found in this work and to that of other countries, which ranges from 5 to 10%33. The EPEC virotype is classified as typical (tEPEC) and atypical (aEPEC). According to the results obtained in this study, only aEPEC strains were identified for both study groups, and these results coincide with what was published by Ochoa et al.34 who affirms that aEPEC is the most commonly associated virotype of diarrhea in children.
The EAEC virotype was found in 14% of the strains for both study groups, data similar to that published by Aslani et al.35 who conducted a study to identify the EAEC virotype in samples of children with diarrhea presenting in 10% of the study population36. Jenkins et al., (2006) out of 500 total samples were able to identify 8% of EAEC.
It has been considered that EAEC is an emergent pathotype37 given that it has been presented in both developing and developed countries as in the case of the United Kingdom37. Furthermore, this pathotype has been associated as one of the causative agents of traveler's diarrhea29.
The genes identified for this virotype were aat and astA, which have been related to the aggregative adhesion phenotype. The astA gene found in greater numbers with respect to the aat gene, in both study groups, which agrees with that published by Jenkins et al.36
Once these genes were identified, the adherence test was performed to evaluate the AA in E. coli strains. In Mexico City, of 17 strains that carried some of the two genes, only one strain showed AA and 12 diffuse adhesion strains. While for S.L.P., of 14 strains evaluated, four strains showed diffuse adherence and four strains AA. It should be mentioned that all the strains that had some type of adherence carried the astA gene. These data show that the astA and aat genes are present in E. coli strains with a not only aggregative adhesion phenotype but are also present in strains of E. coli that adhere to the HEp-2 cells in a diffuse form. These data agree with that published by Jenkins et al.36, who performed the HEp-2 cell adhesion assay and found no relationship between the presence of genes and the aggregative adhesion phenotype.
In relation to the higher prevalence of E. coli virotypes in SLP, compared to the E. coli virotypes identified in the CDMX, this may be due to the living conditions of the population belonging to a former state. According to INEGI, SLP is considered one of the states with the greatest social backwardness, since 53 of the 58 municipalities have a high degree of poverty such as lack of services, education, health, basic services, and spaces deficient housing. This favors that gastrointestinal diseases are a serious health problem for this population.
Another factor of virulence that has recently been considered of great clinical importance has been the production of biofilm in various bacteria, including E. coli12. In the present work, biofilm production was evaluated only in the mobile E. coli strains based on the work of Pratt and Kolter11, Reisner et al.12, and Wood et al.38 who showed that the flagellum is related to biofilm formation. The formation of this was evaluated by two methods: Congo red agar (ARC) and the Christensen method (in tube). By the Congo red method in Mexico City, 70% of the strains were biofilm producers and for S.L.P. 76% with the Christensen method, for Mexico City, 19% of the strains formed biofilm and for S.L.P. 21%. The results obtained showed that, although all the strains evaluated showed mobility, not all of them were biofilm producers. This is why we consider that in addition to the flagellum, there are other factors that are related to the formation of said structure. Other factors related to biofilm formation include cellulose production39, as well as the expression of pili Type 1, which has been considered a determining factor in the first steps in biofilm production40.
The results showed that the most effective method to evaluate biofilm production was the ARC method. These data agree with that published by Serra et al.13 Among the advantages of biofilm formation are resistance by bacteria to environmental conditions, evading the immune response and generating resistance to antibiotics41.
Interestingly, it was observed that strains of E. coli belonging to a certain pathotype carried genes belonging to other pathotypes. In figure 6, we can observe the distribution of genes in strains isolated from both Mexico City and S.L.P. In addition, these data show that the origin of E. coli strains behaves differently when sharing genes between different virotypes, that is, the strains of SLP share genes between different virotypes in relation to E. coli strains of the Mexico City. This heterogeneity can be due to the transfer of genetic material that occurs between the bacteria, which can later lead to a new classification of pathotypes.

Figure 6 ETEC: enterotoxigenic Escherichia coli; EAEC: enteroaggregative Escherichia coli; EPEC: enteropathogenic Escherichia coli; EIEC: enteroinvasive Escherichia coli; EHEC: enterohemorrhagic Escherichia coli. *p < 0.00.
In the antibiogram tests, resistance, and sensitivity to the antibiotics tested were determined. The results showed a greater number of strains of E. coli resistant to different antibiotics in Mexico City compared to strains of E. coli from SLP, with a statistically significant difference for AM, cephalothin, SMX/TMP, amoxicillin/clavulanic acid, cefotaxime, and kanamycin. Regarding the percentage of strains resistant to AM, the results coincide with that published by Jiang et al.42 Ampicillin as well as CF belong to the group of b-lactams, the high resistance of the strains against this antibiotic is probably related to the development of b-lactamases. It has been observed that bacteria that had developed b-lactamases, also show resistance to amoxicillin and some first-generation cephalosporins, as is the case with CF43.
The results obtained in this work showed a higher percentage of resistance to antibiotics of the strains of E. coli belonging to Mexico City, with respect to the E. coli strains of S.L.P. According to Guan et al.44, the resistance can be modified due to geographical location, agricultural management, as well as the levels and types of antibiotics used in the local population. In relation to the sensitivity to the antimicrobials tested, the highest percentage of sensitive strains occurred in E. coli isolated from S.L.P., with statistically significant differences for cefoxitin, SMX/TMP, and amoxicillin/clavulanic acid. This is probably due to better management of antibiotics in S.L.P.
This is the first comparative study performed with diarrheagenic E. coli in Mexico, with stool samples from the federal entities Mexico City and S.L.P. In these strains, both genotypic and phenotypic aspects of the bacteria were analyzed, showing a high prevalence of the ETEC virotype in both populations. This is in contrast to the EIEC and EHEC virotypes, which showed a high prevalence of E. coli strains from SLP, result that is striking since such a high prevalence of these virotypes had not been documented in Mexico. Likewise, a great heterogeneity of genes present in the strains of E. coli was observed, since these strains share genes that allow them to be classified in more than one virotype as the strains from S.L.P. being more heterogeneous
Both the biofilm formation and the adhesion to HEp-2 cells were presented in greater numbers in strains of S.L.P. All these factors related to the virulence of E. coli, allow us to consider that the strains from S.L.P. are more virulent than the strains isolated in the Mexico City.
Regardless of the fact that E. coli is one of the most studied bacteria, the present work showed that this bacterium still has a lot of information to contribute.











 nova página do texto(beta)
nova página do texto(beta)





