Abbreviations and acronyms
AIT |
allergen immunotherapy |
AR |
allergic rhinitis |
DCs |
dendritic cells |
fVAS |
Faces Visual Analog Scale |
HDM |
house dust mite |
IDIT |
intradermal immunotherapy |
SCIT |
subcutaneous immunotherapy |
SLIT |
sublingual immunotherapy |
TNSS |
Total Nasal Symptom Score |
Background
Allergen immunotherapy (AIT) is a more than a century old therapeutic technique1,2 involving the administration of increasing concentrations of allergenic extracts. It has been shown to reduce symptoms and medication usage while improving patient’s quality of life.3,4 A well-known unique feature of AIT is the modification of the natural course of allergic diseases, inducing sustained long-term effects persisting years beyond treatment discontinuation.2,3,5 In tropical/subtropical areas of the world with high levels of relative humidity all year round, domestic mites are main sources of sensitization;6,7,8,9,10,11 perennial symptoms prevail and pollinosis, for the most part, is a non-relevant clinical issue.9,10
High dose aqueous subcutaneous immunotherapy (SCIT) is a validated and effective route of allergen administration for the house dust mite (HDM) as for pollen allergies.12,13,14 For HDM, 7 micrograms of major allergens per maintenance subcutaneous injection of 0.5 mL is considered an effective high dose;12 general agreement exists, however, around the clinical ineffectiveness of very low allergen dosing.15,16 Injection allergen treatments (SCIT) require a build-up phase, a demand for frequent injections and risk of systemic reactions/anaphylaxis. Other routes of administration have been developed,17 like the sublingual route (SLIT), to circumvent some of above inconveniences, particularly in children; however, when the issue of compliance has been addressed, it appears that SLIT is not favored over injected SCIT.18
The intradermal injection route,19 may offer the possibility of using lower allergen dosing while maintaining clinical efficacy. Successful intradermal immunotherapy (IDIT) approaches with aqueous extracts for grass pollen allergies20,21,22 23 and for bullous reactions from mosquito bites, have been reported.23,24 However, later findings from a grass pollen allergy intradermal immunotherapy (IDIT) study have raised significant controversies around it´s efficacy.25 A recent book chapter on AIT by Nelson12 makes no mention of this route of administration and to our knowledge there are no reports of IDIT use with the HDM.
Antigen presenting cells (Langerhans cells), a unique cellular element in the development of allergen tolerance induced by antigen injections, are found in significant numbers in the epidermis. These cells are distinguished from dermis dendritic cells (DCs) by the presence of the CD1a marker, whereas dermis DCs show positivity for FXIIIa.
The intradermal route may allow for a more direct and efficient allergen processing and hence a need for lesser allergen volumes and concentrations, when compared to high dose SCIT administration. Avoiding the need for a build-up phase, minimizing the risk of a possible systemic reaction and savings likely derived from lower allergen volume/concentrations are endeavors worth looking into. Our institution, caring for a low-income population, is highly aware of costs containments needs while keeping the quality of medical care.
We carried on a real-life and proof-of-concept pilot study to test the efficacy of a lower dose HDM major allergens via the intradermal route (IDIT), in pediatric patients with allergic rhinitis symptomatic on exposure to house dust.
Methods
Patients
Immunotherapy-naive patients with allergic rhinitis (AR) attending a pediatric allergy clinic in a hospital setting caring for a low-income population were offered this treatment modality. The protocol was approved by the Institutional Review Board (IRB) of Hospital San Juan de Dios, Caracas, Venezuela, and the study was carried out from July 2016 till November 2016, with patients and families having to sign an informed consent form.
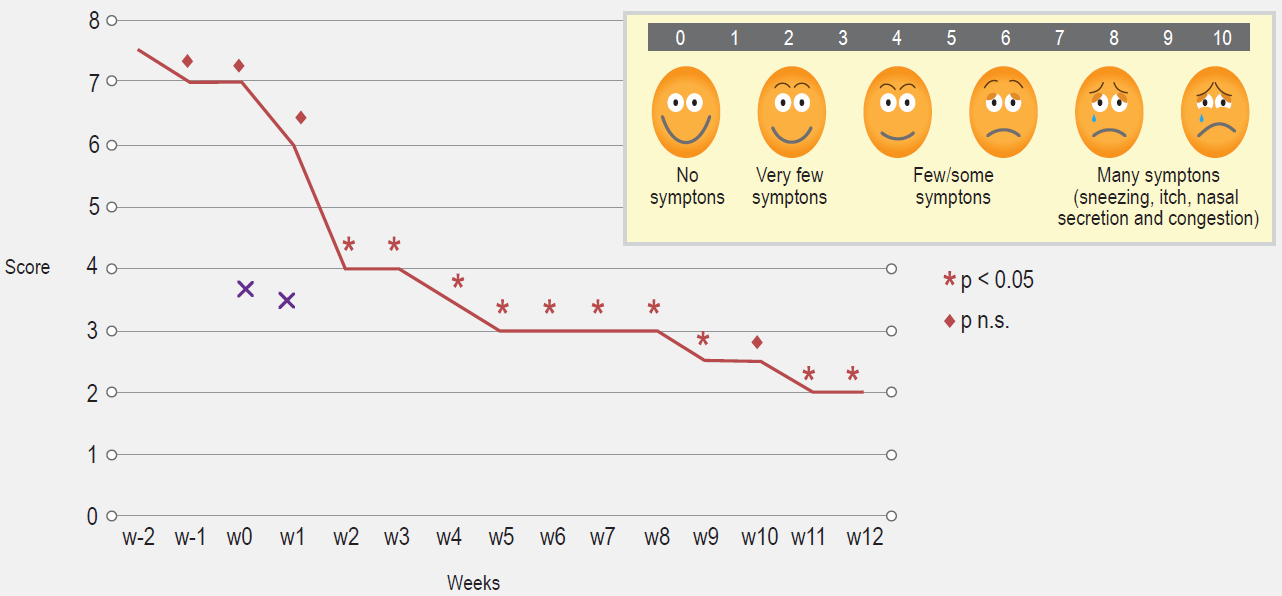
Patients with perennial symptoms of allergic rhinitis (AR) such as recurrent sneezing, itching, rhinorrhea and nasal obstruction for at least of 2 years duration were selected. Patients were instructed on filling out a daily symptom score on a scale ranging from 0 (no symptoms) to 3 (severe symptoms) for each of the four clinical manifestations (sneezing, itching, rhinorrhea, nasal obstruction), as depicted in patient´s diary. A minimum of 100 score/points registered for the Total Nasal Symptom Score (TNSS),26 during the previous 2 weeks recruitment period were needed for inclusion (compatible with moderate rhinitis), as well as a clear history of symptom exacerbations when exposed to house dust (house dust disturbances like sweeping, bed cleaning and the like). A Faces Visual Analog Scale (fVAS) score was concomitantly completed.27,28 The TNSS and fVAS (once comprehension and subsequent reporting from patients/families was achieved to authors satisfaction), were to be filled out at home, with help from parents.
It is generally recommended to employ a combination of symptoms/VAS scores along with allergy medications usage in the evaluation of any intervention on allergic rhinitis;26 in our case, medications usage was not considered in the analysis because this low socioeconomic population has almost nil access to medications,29 something that at the end made for more plausible results. Daily TNSS/fVAS scores for two weeks (baseline data) were to be recorded in the evening (around 8 pm) while omitting any allergy medications, allowing capture of symptoms/signs as they happened in a “real life” situation. Once treatment began, the TNSS/fVAS diary card was filled out at home the day before the weekly injections (12 injections), along with a medication (s) usage record (on an only as needed basis). School age patients were preferred, for full comprehension of symptoms and ability and willingness to fill out the symptoms scores (personally or by proxy). Detailed instructions/education was given by one of the investigators (AV) on how to translate symptoms/signs reliably into score numbers, on a scale from 0 to 3. Patients/families had to be proficient in symptoms-signs recognition as well as in their numerical interpretation, before admission into the study. Furthermore, easy access to the Hospital and a home/cell phone were required for compliance reasons.
Patients receiving intranasal or oral steroids within 1 month prior to the study and/or AIT use ever, as well as passive tobacco exposure, allergic respiratory symptoms triggered by home pets (dogs, cats) regardless of their skin test positivity, or other chronic diseases such as cystic fibrosis, heart diseases, respiratory illnesses (sinusitis, adenoid hypertrophy, symptoms compatible with obstructive sleep apnea, significant septum deviation, or nasal polyps) that might interfere with symptoms interpretation, were excluded from the study. Investigators were always available by phone to answer questions. The use of a control group was discarded (“histamine sham injections”) from the beginning by recommendation of the IRB. All patients received a written pictorial hand-out material detailing eviction house dust measures, as part of our clinic routine work-up.
During the pre-treatment baseline phase, thrice weekly phone calls were made for reinforcing above abilities; thereafter, weekly SMS text messages were sent to participants reminding patients/families to bring the filled TNSS diary at their weekly allergy shot appointments; a new TNSS card was to be dispensed then. Medication use, like antihistamines/antileukotrienes/intranasal corticosteroids, was discouraged during IDIT treatment, and allowed on a as needed basis only; if so, it had to be properly registered in the dairy.
Treatment compounding
A 50 % glycerinated 30 mL extract of a Dermatophagoides pteronyssinus/ Dermatophagoides farinae (Dp/Df) mixture from Greer Labs, Lenoir, NC, USA, and labeled as “stock solution” (lot number # 308809, 10.000 AU/mL: 5,000 AU Dp/5,000 AU Df, expiration date: June 2019) and a Blomia tropicalis skin test extract labeled also as “stock solution” (BIAL Laboratories, Vizcaya, Spain, 30.000 DBU/mL, skin test phenol-glycerinated extract, lot # 14125; expiration date: November 2016), were used for skin testing as well as for compounding the allergenic material employed in the IDIT injections (following ACAAI´s Allergen Immunotherapy Extract Preparation: Physician Instruction Guide).30 This Dp/Df mixture from Greer Labs contains approximately 62 micrograms/mL of major allergens,12 corresponding roughly to a 1/100 dilution w/v. Likewise, for the Blomia tropicalis “stock solution” a 1/100 w/v dilution was assumed. For the Blomia tropicalis allergen only the skin test material is commercially available in our country. A volume of 0.05 mL of each of such extracts was added to a 30 mL flask of phenol/saline/albumin, as dispensed by Greer Labs for enhancing the stability of very dilute extracts31,32 (lot # 231416, expiration date: June 2017). Each 0.05 mL volume from this newly prepared flask was used for the IDIT injections containing approximately 8.3 AU of Dp/Df or 0.05 micrograms (5 nanograms) of HDM major allergens. This 5 nanograms concentration of HDM allergens is quite like the nanograms concentrations employed in previous reports for grass pollen IDIT;22,25 for the Blomia tropicalis allergens a 2.5 DBU/0.05 mL was correspondingly estimated. This 30 mL multiple-injection flask allowed for ease of operation in our weekly busy clinic; it was properly stored at 4-8 °C and taken out of the refrigerator once a week. For sake of simplicity and reproducibility, a volume of 0.05 mL was adopted for this compounding needs as for the IDIT injections: this is the minimal volume that can be reliably measured with disposable 1 mL syringes.
Skin tests
Prick tests were performed using the standardized Hollister-Stier Lancetter® and reactions were considered positive if papules were > 3 mm than the negative control (table 1). No demonstrable dermographism was observed in any of the patients and these lancets make for almost no skin irritation. Papules length and width were read at 15 minutes and graphically outlined on a scotch tape-for transport on patients chart-and proper measurement, as per Dreborg.33 Besides the Dp/Df and Blomia extracts (“stock solutions”) used in prick testing, additional prick skin tests with other inhalant extracts (cat, dog, cockroach, grass mix, mold mix from ALK-Abello®, Madrid, Spain) were also performed, read at 15 minutes and recorded, along with Histamine 1 mg/mL and a negative glycerosaline control. Furthermore, freshly prepared 50 % glycerin serial dilutions for prick skin tests (SDSPT) were prepared from these “stock solutions”, ranging from a 1/100 w/v to 1/1.000.000 w/v. For better readings these prick tests were placed in patients forearms volar surface-in duplicate-(with a variation coefficient of 15 %) before and after study completion. Antihistamines, if used, had to be omitted 5 days prior to testing and papules were read at 15 min and graphically transported, as above.
Immunotherapy treatment
Patients received weekly 0.05 mL ID injections in the middle and external area of arms, during our routine weekly clinic gatherings; disposable 0.3 mL/31 G sterile syringes (Beckton-Dickinson) were used for an appropriate visual estimation of the volume (0.05 mL) to be injected by our trained personnel. A "peau d’orange" papule was to be formed (as performed in routine PPD skin test) and small dimples visualized, without any blood drainage; arms sites were weekly alternated. Experience demonstrated that a volume of 0.05 mL, when intradermally injected with this technique makes for a papule of 0.5 cm2. Patients remained at study site for at least 30 min after injections, and an antihistamine according to weight was administered 1 hour before the weekly shots at our clinic. For the first and last injections, 5 previous days without antihistamine use were required allowing for papules and erythema from the injection site to be properly measured, at 15 min, and registered as above. Allergy treatments were given in a clinical area with full resuscitation facilities. Adverse local or systemic reactions were noted. The 30 mL compounded flask may dispense for up to 600 injections.
In vitro tests
A 3 mL of a blood sample from the antecubital fossa, on a plain tube without anticoagulant, was drawn before and after study completion; two patients (families) refused to have blood drawn but agreed to go along with the rest of the study. Blood was centrifuged at 5000 rpm and sera saved at -20º C, until analyzed. Minividas (Biomerieux, France) was used for total IgE determinations (kU/L), according to manufacturer’s specifications. For specific levels of IgE and for the IgG 4 for Blomia and a Dp/Df mixture, the PHADIA CAP 250 Thermo-Scientific ® automated method was employed, under manufacturers specifications.
Results
Symptoms
As can be seen in Figure 1, TNSS were found to be significantly lower, when compared to pre-treatment values. Significant symptom improvements were observed after one month of treatment. The same goes for the VAS scores (Figure 2). Except for the day of IDIT injections, no use of antihistamines for symptoms control were reported before or after immunotherapy treatments, except for one patient and on a single day (their use was not encouraged); also, no fever episodes with symptoms compatible with those of upper respiratory infections, were noted. Hence, these results are likely attributable to the immunotherapeutic intervention.
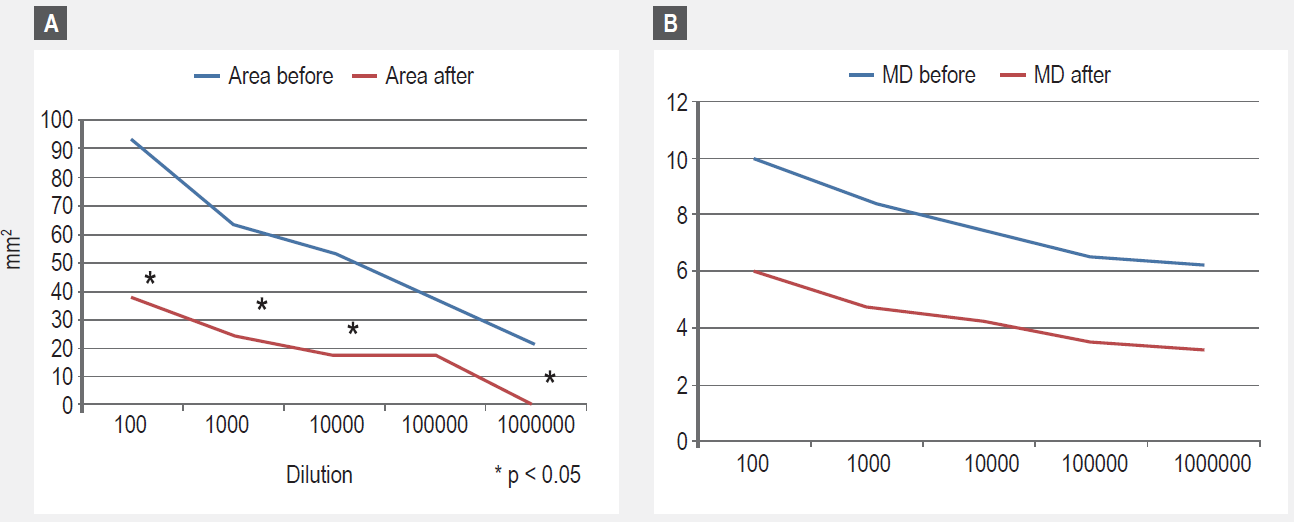
Serial dilution skin tests
In Figure 3, the results of skin test titration expressed as combined wheal size diameters are presented. A significant decrease, when initial and post -treatment measurements are compared, was observed.
In vitro tests
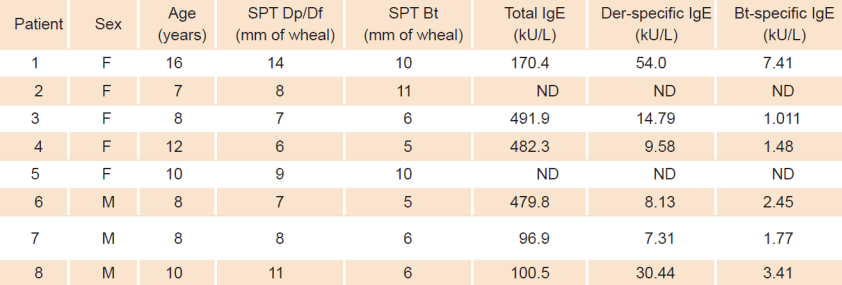
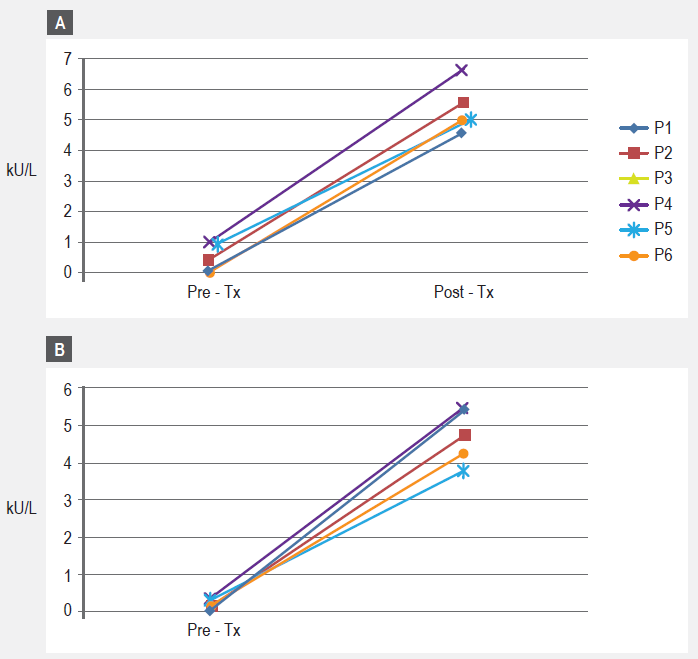
For the six patients who agreed to have blood drawn, pre-treatment total IgE showed values ranging from 96.9 kU/L to 482.3 kU/L (mean 303.6 IU/mL) (table 1). The pre-treatment specific IgE profile of our patients showed negative (< 0.35 IU/mL) or negligible values for epithelia (cat, dog) and cockroach, coinciding with skin test results (data not shown). As for specific IgE results to the DP/DF mite mixture, values ranged from 7.31 IU/mL to 30.44 IU/mL (mean 20.7 IU/mL) and values for Blomia tropicalis ranged from 1.01 IU/mL to 7.41 IU/mL (mean 2.9 IU/mL) (Table 1 and Figure 3). Significantly increased post treatment IgG4 responses were observed for Blomia tropicalis only (Figure 4a). A similar, but not statistically significant trend was observed for the mite mixture IgG4 responses (Figure 4b).
Discussion
We observed TNSS/fVAS scores to be significantly decreased when compared before and at 3 months after treatment, suggesting a favorable impact of IDIT on children with moderate to severe allergic rhinitis sensitized to Dermatophagoides and Blomia tropicalis allergens, and symptomatic on exposure to house dust. Our weekly clinic routine employs SCIT increasing concentrations (build-up phase) of aqueous extracts of HDM major allergens and Blomia tropicalis, till a maintenance dose is reached; keeping the same lesser volume/allergen concentrations (0.05 mL) every week allowed for avoiding the bothersome dose increases at injection sites (build-up phase). Furthermore, this seldom reported technique employing a # 31 G needle disposable syringe seemed no more painful that the reported standard # 27 needle SCIT administration.
AIT has been part of allergist’s armamentarium1,2,3,4,5 for over a hundred years; well controlled studies have confirmed the effectiveness of high dose injection therapy (High Dose Aqueous SCIT) in both seasonal and perennial allergic rhinitis12,13,14 Notwithstanding, it seems well-established that low dose SCIT is clinically ineffective15,16 .In our tropical humid environments respiratory allergies tend to be perennial, with HDM/Blomia tropicalis standing out as major allergens, as in many other developing and tropical areas of the world;10 pollens, as evidenced in our patients´ sensitization pattern, are of no major concern. In many Latin American countries34 and likely in other nations,35 cost remains a key issue impinging on the AIT access/adherence for patients with respiratory allergies. IDIT has studied, with its inherent low dosing (volume and concentrations) and no need for a build-up phase, explored possible cost-effective alternatives for low income populations.
This pilot real-life “proof of concept” efficacy study allowed for a 140-fold less HDM major allergens injections, compared to the 7 micrograms clinically effective high dose recommended for SCIT12 (per maintenance dose of 0.5 mL). Our patients received only 5 nanograms (0.05 micrograms) of HDM major allergens/0.05 mL injection/week, all throughout the 12-week study period (a cumulative dose of 0.6 micrograms/12 weeks). Previous grass allergens IDIT studies that employed a 7 ng dose22,25 served as an orientation for our HDM concentration choice; no such information exists for Blomia tropicalis, but we followed the same dilution criteria for HDM injections: each 0.05 mL IDIT weekly injections contained 2.5 DBU of the Blomia tropicalis allergens. On the other hand, the volume of weekly injections employed was reduced 10 times (0.05 mL IDIT vs 0.5 mL SCIT) and employing the 0.3.mL/31 G needle syringes allowed for a fine tuning when performing the “peau d’orange” technique bleb.
There are very few studies employing the IDIT technique19,20,21,22,23,24,25 and none, known to us, with HDM allergens. Doses employed in previous reports of IDIT with grass pollen were guided by the suppression of late cutaneous reactions,22,25 with cumulative doses reaching 1000 fold less allergen vs conventional SCIT over a year´s time (nanograms vs micrograms); furthermore, in these studies, injections were given every two weeks for three months and spaced out afterwards.22 Our multiple-injection 30 mL vial flask accommodated particularly well into our weekly busy clinic operation. Our compounding preparations were guided by the minimal volume that could be accurately measured with conventional disposable syringes, making this endeavor reproducible and as “real life” as possible. We had no severe allergic systemic reactions, while mild local reactions as treatment progressed, diminished in duration and intensity. All were well tolerated as per reports of patient/families.
A pertinent key issue rests on the means used to demonstrate clinical efficacy. In allergic rhinitis, a TNSS yet, remains a paradigm.26 One of our investigators (AV) suffers from perennial house dust allergic rhinitis and is aware of symptoms and signs of this clinical entity. He oversaw educating patients/families on how to proceed in the translation of symptoms/signs into a numerical score (0-3). This likely made the TNSS a more reliable instrument of data collection, and the same goes for the fVAS score. We started to see significant improvements at around the first month of treatment (Figures 1 and 2). A reinforcement of adherence with SMS texting and phone calls undoubtedly helped. Also, our low socioeconomic level population has none or little access to allergy medications;29 besides, their use was not encouraged during the study. We were not able to check upon patient/family compliance with environmental control measures, making impossible ascertaining the role of eviction house dust measures in our results.
Immunological evidence of a response to the treatment came from the SDSPT results as well as from the significantly increased levels of specific IgG4 to Blomia tropicalis; notwithstanding, an increased non-significant IgG 4 trend for the HDM mixture could be appreciated. Such early impact on cutaneous responses (SDSPT) is also found from cluster immunotherapy treatments with HDM allergens, as well as with other inhalants.36,37,38,39,40 For practical reasons we did not measure the delayed cutaneous responses although other reports have considered that it could be evidence for symptoms improvement.22,41 We did measure the papule size at the site of the ID injection (papule read at 15 min) the day of the first and last injection, finding a non-significant diminution. Our immunologic findings correlated with improvement in symptoms, though we acknowledge the controversy presently surrounding this issue;25 it remains a conundrum if intradermal immunotherapy might foster improvements or, as demonstrated for grass pollen, aggravates allergic symptoms.25 The small size of our pilot study precludes any definitive comparison with previous reports.
In conclusion, albeit the small sample size and lack of a control group, our findings suggest that this little explored route and technique of administration, for HDM allergens, should be further pursued. We are in the process of expanding our study, with a larger number of patients and longer follow-up, to better discern the place of such a low dose/low volume IDIT technique. We hope to encourage immunotherapy research centered on sound cost-containment alternatives. This endeavor follows our long time efforts42,43,44,45 in the design of treatment strategies for allergies and asthma suitable for the underprivileged contexts found in developing nations. If findings from the present study are reproducible, perhaps more patients could afford needed AIT treatments.











 nova página do texto(beta)
nova página do texto(beta)