Background
Atopic dermatitis or allergic eczema is a chronic disease that can be severe and can cause a significant deterioration in the quality of life. In severe cases, it may have even greater psychosocial impact than other chronic diseases such as diabetes mellitus or myocardial infarction.1,2
This disease usually begins before the age of two but can occur at any time of life and although in most patients usually symptoms disappears before puberty may extend into adulthood. International consensus gives several recommendations for the treatment of dermatitis, recognizing as a pillars of treatment hydration, avoidance of irritants and topical pharmacotherapy.3,4,5 However, not always adequate control is achieved with these measures and some patients requiring more aggressive treatment such as phototherapy or systemic immunosuppressive drugs. Why some patients respond better than others to drug treatment is under study and some environmental factors seem to influence the type of clinical response.
There are few Latin American cohorts focused on allergic diseases6,7,8,9 and none of these cohorts has as principal focus dermatitis. Studies in this region are important because there are disparities among some phenotypes, such as IgE sensitization and prevalence of atopic eczema, when compared to those observed in industrialized countries.9,10
In this study, based in the TECCEMA cohort (Tropical Environment Control for Chronic Eczema and Molecular Assessment), developed in a tropical region with conditions that favor exposure to high concentrations of allergens,11,12,13 we evaluated the clinical control obtained following the recommendations from various guidelines and international consensus on dermatitis in real life conditions, using objective and subjective assessment instruments scales.
Methods
Location and study population
The Ethic Committee of the University of Antioquia (Medellín, Colombia) approved this study. This study was done in a community based cohort population conformed in a tropical environment for a prospective follow-up and collection of epidemiological data and biological samples. Medellin is the principal city of Aburra Valley which is a tropical region from Colombia (6° 14’ 41″ North, 75° 34’ 29″ West) with an average annual temperature of 24 °C and 66 % of relative humidity. 40 % of patients enrolled in the cohort are poor according to governmental indexes that assess type of housing, overcrowding (three or more people per bedroom), access to basic services, income (minimal wage 250 dollars per month) and school attendance. According with this socioeconomic stratification 70 % of the population is poor. All study participants shared the same environmental conditions. The genetic background of this population resulted from racial admixture between Native Americans, Spaniards, and at lower rate of (< 10.9 %) of African ancestry.14,15
Study design
The TECCEMA cohort is a prospective observational study (Figure 1). Patients with diagnosis of dermatitis attending different public and private health centers were screened and recruited between December 2011 and December 2013. We included patients over 3 years of age with clinical history of AD for more than 1 year, with chronic eczema. During the first visit, dermatitis was diagnosed when all the following criteria were present:16
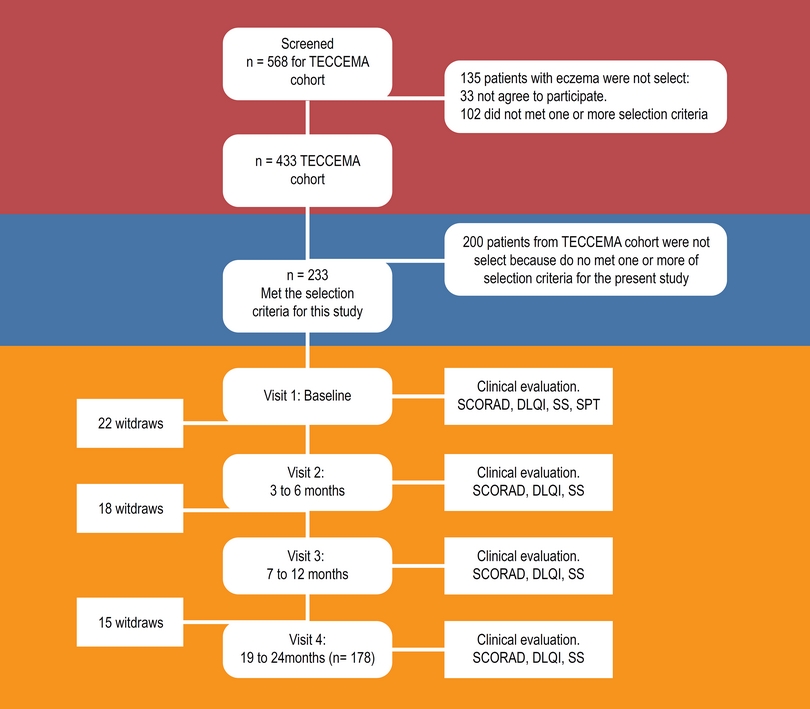
Figure 1 Flowchart. TECCEMA, tropical environmental control for chronic eczema and molecular assessment.
Evidence of itchy skin/pruritus.
Visible eczema.
Typical morphology and distribution including facial, neck and extensor involvement.
Dry skin.
Considering that in this community other causes of chronic itchy skin are common (scabies, insect stings, helminth induced rashes, etc.) we included only patients with SCORAD over 8 points and for the same reason the diagnosis always was done by dermatologist or Allergist. Atopy was evaluated by serological measure of specific IgE and/or skin prick test (SPT) according to GA2 LEN recommendations, using the most prevalent extracts in the region.17
Severity evaluation
Severity was assessed with SCORAD (Scoring Atopic Dermatitis) at the baseline and during the follow-up. Dermatitis was ranked as severe (> 40 points), moderate (16 to 39), or mild (< 15). Additionally, in patients with baseline SCORAD > 15 points was considered “complete control” when SCORAD was less than 15 points for at less 6 months.
Quality of life impact
Among the quality of life questionnaires, the DLQI (Dermatology Life Quality Index) was selected for this study since it was previously validated in Colombia.12 We considered that patient had complete control when DLQI was ≤ 5 points “partial control” 6 to 10 points and “no control” > 10 points. Copyright statement of DLQI described on the website (www.dermatology.org.uk) was fulfilled.
Additionally, each patient or their parents answered a subjective evaluation consisting of 3 questions (each question scale 0 [mild] to 10 [severe]) assessing general severity perception of pruritus, eczema and social impact (Subjective Score: SS);18 the average score of the three questions was expressed as a percentage. We considered that patient had complete control according to SS when was < 20 %, partial control 21 to 60 % and no control > 61 %.
SCORAD, DLQI and SS were repeated during the follow-up.
Guideline recommendations
We organized treatment registers according to the European Task Force on Atopic Dermatitis (ETFAD) scale (Figure 2).4,5 ETFAD divide the phase of treatment in baseline (skin hydration, avoidance of irritants), phase 1 (topical steroids, topical calcineurin inhibitors), phase 2 (phototherapy) and phase 3 (Hospitalization, systemic immunosuppression). Phase 1 is recommended for patients with SCORAD < 15, phase 2 for SCORAD 16 to 40 and phase 3 for SCORAD > 40. Patients in phase 2 or 3 usually are resaving topical treatment recommended in phase 1 and baseline. For descriptive propose we divided treatment in step 1 (baseline and step 1) and step 2 (step 1 + phase 2 and/or 3).
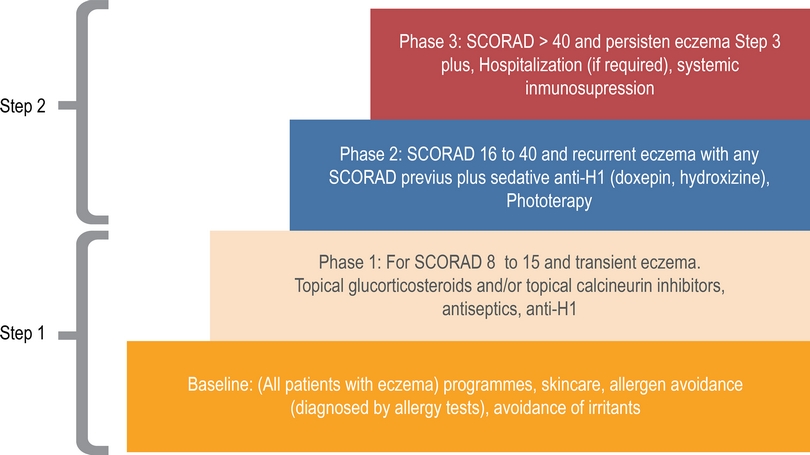
Figure 2 Management flowchart according ETFAD guide. We divide this management in phase 1 and phase 2.
Step 1 include environmental and diet restrictions, oral antihistamines, emollients, topical steroids, and topical calcineurin inhibitors. Doses, frequency of use, and relative potency of drugs were registered.
Step 2 include phototherapy, immunosuppressive drugs and hospitalization. In most cases this therapy was apply in patients with severe SCORAD but in some patients with moderate or mild was required for recurrent acute exacerbations. Doses, frequency of use, and relative potency of drugs were adjusted according age of patients and severity.
As principal aim, we evaluate how many patients achieved clinical control with the guidelines recommendations. TECCEMA staff did not intervene in medical decisions but adherence and changes in treatment indications were registered.
Data analysis
Statistical analyses were done using SPSS 21 (Chicago, IL, USA). Frequencies and descriptive statistics were calculated at baseline, and during each visit of follow-up. Outcomes of drug effectiveness ware evaluated. Use of step 1 was continuous during follow-up in all patients with little changes in time. For the other side, some patients began the study with step 2 and other start step 2 during the follow-up, so the number of patients with step 2 changes during follow-up and only patients with at less 3 months with step 2 were included for efficacy analysis.
Chi-square was used to analyze the differences between dichotomic variables. For contingency tables with less than 10 cases in any cell, the Fisher’s exact test was used. To analyze the clinical response according each step, multivariate analyses were performed and 95 % confidence interval were calculated.
Results
Demographic characteristics
Four hundred thirty-three patients were included in the TECCEMA cohort and 233 were selected for the present study. 178 (76 %) finished the follow-up (mean age 8 range 3 to 41 years); 114 females and 119 males. The sociodemographic characteristics of the excluded patients were similar to those that continued in the study. Antecedents of allergic diseases were similar between excluded and non-excluded patients and did not influence the willingness to participate. Fifty-five patients were lost during follow-up (Figure 1). Reasons for exclusion were moving out of city (n = 14), loss of contact (n = 24), incomplete clinical record (n = 10), family declined (n = 6), death (n = 1). Most of the patients lost were in the first year (n = 40). All patients had similar environmental and living conditions. As confirmed by questionnaires and medical records, the beginning of eczema in 64 % of patients was between 0 and 2 years of age; 24 % between 3 and 5 and 12 % over 5 years. Asthma and rhinitis were the most common comorbidities (75 %) and dust mite allergens the main sensitizers (93 %) (Table 1).
Table 1 Baseline characteristics in all patients and according to SCORAD severity
| Baseline characteristics | Atopic dermatitis | |||||||
|---|---|---|---|---|---|---|---|---|
| All patients | Mild | Moderate | Severe | |||||
| Patients number | 233 | (100 %) | 53 | (22 %) | 116 | (49 %) | 64 | (27 %) |
| Age (range) | 8 | (3-41) | 9 | (3-38) | 8 | (3-41) | 7 | (3-40) |
| Gender (female) | 114 | (48 %) | 26 | (49 %) | 58 | (50 %) | 30 | (46 %) |
| Asthma/rhinitis | 175 | (75 %) | 26 | (49 %) | 91 | (78 %) | 58 | (90 %) |
| Sensitization | 219 | (93 %) | 48 | (90 %) | 109 | (93 %) | 62 | (96 %) |
| SCORAD (range) | 33 | (8-108) | 12 | (8-15) | 33 | (16-39) | 51 | (40-101) |
| SS (range) | 85 % | (89-100) | 60 % | (40-94) | 84 % | (63-100) | 93 % | (80-100) |
| DLQI (range) | 14 | (3 -30) | 8 | (3-30) | 14 | (8-30) | 21 | (11-30) |
SCORAD, scoring atopic dermatitis; SS, subjective score
Pharmacotherapy management
We divided phases of pharmacotherapy recommended in ETFAD guideline in two steps (Figure 2):
Step 1
Humectants creams: All patients receive moisturizing creams with changes in the type during follow-up. 189 patients received during some moment of the follow-up baseline or occlusive creams, but 168 (88 %) of them suspended by burning or discomfort. For the contrary, patients who receive emollients or no occlusive moisturizers (n = 200) had better adherence (82 %), continuing with them during follow-up.
Antihistamines: From the 178 patients who received for at less 3 months antihistamines, 48 (26 %) reported a strong sedative effect. We did not observe significant different between patients with or without antihistamines according to general characteristics or SCORAD.
Topical steroids and calcineurin inhibitors: All patients received one or more topical immunosuppressor during the follow-up. Most patients (n = 222, 95 %) tolerated the different topical steroids or calcineurin inhibitors. 11 patients reported a burning adverse effect, 9 with calcineurin inhibitors and 3 with steroids. The frequency of administration, dose and concentration was adjusted according to age of patients and severity.
Irritant restrictions: All patients during baseline-received instructions to avoid possible irritants like fragrances, pets (if there had IgE sensitization to pets, n = 112) and environmental control measures for dust mites. 168 (n = 72 %) patients recognized that they frequently fail avoidance measures. The principal reasons were “difficulty complying” (n = 98) and “not wanting” (n = 70). 162 patients suspected that one food worsening symptoms therefore they did restricted diets. The foods most frequently reported were egg (n = 82), corn (n = 61) and pork (n = 56). During skin test, most of them were negative for the food that they suspect (95 %, data no shown).
Step 2
In most cases step 2 was apply in patients with severe SCORAD, but in some patients with moderate or mild was also required for recurrent acute exacerbations.
Phototherapy: 56 patients (24 %) received phototherapy. The mean sessions were 90 (3 to 140) 12 patients suspend because local side effects like irritation and worsening of eczema.
Hospitalizations: Twenty-eight hospitalizations were documented in 15 patients with severe SCORAD, with a mean of clinical care of 3 days (range 2 to 14). The principal reasons were systemic cutaneous infection (n = 14), eczema over 90 % of body area (n = 12). Two patients were hospitalized to ensure the realization of the exams and the start of systemic treatment.
Systemic steroids: During the two years of follow-up, a total of 64 cycles of oral steroids were prescribed; 12 in 8 patients with moderate SCORAD with acute exacerbation and 42 in 20 patients with severe SCORAD.
Immunosuppressive drugs: A total of 97 patients (41 % of 233) required one or more immunosuppressive drugs during follow-up; 60 patients with severe SCORAD, 33 with moderate and 4 with mild (this 4 for intense itching with high impact on the DLQI and SS over 6 months). The most common immunosuppressive drugs prescribed was cyclosporine (n = 83) follow-up by phototherapy (n = 43), mycophenolate mofetil (n = 18), methotrexate (n = 14), omalizumab (n = 12) and azathioprine (n = 9). Fifty-three patients changed immunosuppressive drug at least once: The main reasons for the change of immunosuppressive drug were reversible adverse effects (43 %) and lack of clinical response (41 %). Four patients had irreversible adverse effects associated with immunosuppressive. When we evaluated the therapies of step 2 separately, we observed some different: Phototherapy had the highest frequency of adverse events but have a faster clinical effect. After 12 months Cyclosporine had the highest reduction in SCORAD. 26 % received additional treatments (homeopathy, acupuncture and others) not included in the recommendations of the guidelines.
Control of eczema according to SCORAD
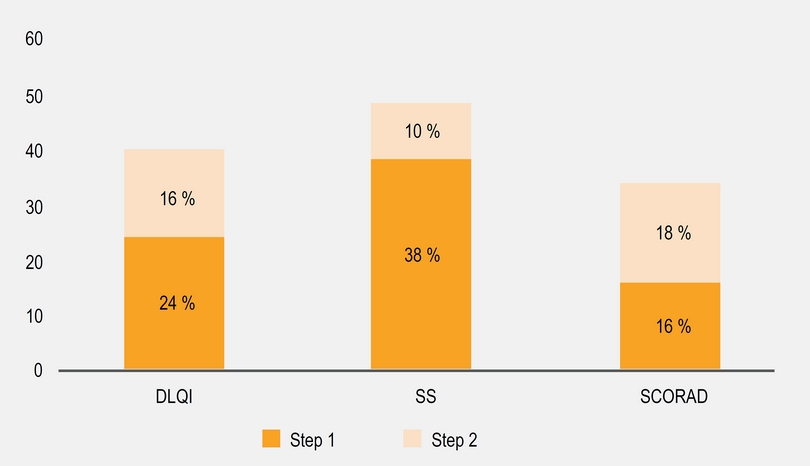
As we described before, most patients received a combination therapy including skin hydration, avoiding irritants and topical drugs. During follow-up, we observed a significant reduction in SCORAD after 6 months (Figure 3a) in most patients. We did subgroup analysis according to the SCORAD and we find that moderate group contributed with the 65 % of the reduction observed. Between patients with baseline moderate or severe SCORAD, only 33 % achieve a SCORAD < 15 after 2 years with step 1 or 2 (Figure 4). 7 % of patients with baseline mild SCORAD finished the last 6 months of follow-up with SCORAD over 15 points.
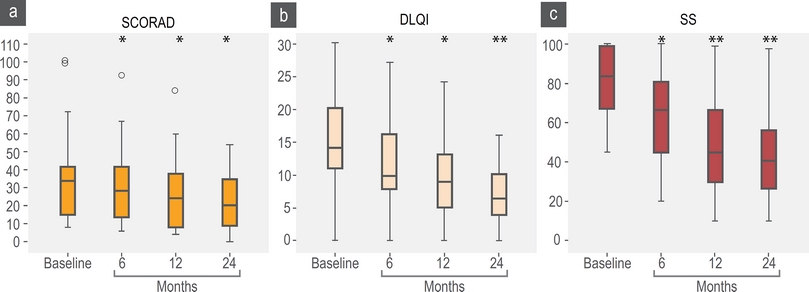
Figure 3 SCORAD, DLQI and SS scores. SCORAD baseline mean 33 points (percentile 25-75; 15-41); 6 months, mean 29 (14-41); 12 months, mean 24 (8-37); to 24 months, mean 21 (9-34). DLQI baseline, mean 14 (11-20); 6 months, mean 12 (8-16); 12 months, mean 9 (5-13); 24 months, mean 7 (4-10). SS baseline, mean 85 (67-99); 6 months, mean 62 (45-80); 12 months, mean 48 (30-66); 24 months, mean 41 (27-56). *p < 0.05, **p < 0.01
Impact in quality of life according DLQI and SS
We observed a significant reduction in DLQI and SS scales (Figures 3b and 3c) and DLQI and SS had direct significant correlation (R = 604 p = 0.03) but not with SCORAD. A higher proportion of patients reported a “completed control” according DLQI and SS over SCORAD (Figure 4). We observed that some patients with severe (n = 20) and moderate (n = 33) SCORAD, reported a DLQI < 5 and SS < 20 % without a significant change in the SCORAD: When we explore the SCORAD in these patients, they had no change in points from the fields type of injury and length of eczema but they had a significant reduction in subjective perception of severity of pruritus and sleep discomfort.
Discussion
The principal results in our study were:
We created prospective cohort of patients with dermatitis in the tropics with environmental and socioeconomic conditions particular. This cohort could help to understand how these factors influence the development of dermatitis and the clinical response to pharmacotherapy.
We observed that recommendations from international guidelines have a significant impact reducing the severity of symptoms, improvement the quality of life and subjective perception. However, about half of patients do not reached complete control.
Currently, there are many guidelines regarding the management of dermatitis and most of the recommendations are based in expert opinion or case-control studies conducted in Europe or USA in subtropical areas. Little has been studied about the clinical impact of these recommendations in the real life and less in the population of tropical region. We observed in a tropical region that most physicians meet the guideline recommendations and most patients had no problems in complying this management but 26 % of patients additional to the conventional treatment use alternatives therapies and most patients tolerated moisturizes but not occlusive creams. This could be explained by the environmental conditions in tropics; occlusive creams in high temperatures could produce severe discomfort and the use of alternatives therapies could be due for cultural and popular beliefs but also for a perception of little improvement with conventional therapy.
Most patients tried to apply environmental control recommendations. The avoidance of irritants is widely recommended but difficult to meet for some allergens: In the Aburra Valley, one of each three households have a pet and mites allergen exposure is almost universal because warm and humid environment facilitate the growth of a diverse fauna and the perennial exposure to high concentrations of their allergens.10,12 Similarly, overcrowding of patients secondary to low income facilitates the accumulation of allergens and other irritants.9,13 Most patients associated worse of symptoms with foods, but according to our results foods restrictions for the foods suspected by the patients had in most of them little or no clinical impact (data no shown).
Particular conditions in each environment make that certain therapeutic measures are preferred over others. Despite that phototherapy is widely recognized as an effective treatment for moderate or severe dermatitis, there are few centers in our community that can perform this therapy, so other immunomodulatory therapies were more frequently used. Most patients present an important reduction of symptoms with pharmacotherapy recommendations, however less than 50 % of patients reached the target of control according to SCORAD, DLQI and SS together. As noted above we believe that tropical environmental conditions could influences these results specially humidity and weather, but other factors like genetic ancestry and lack of more effective therapies could explain at less in part these results. Recent studies suggest that monoclonal antibodies as dupilumab, etanercept, could be an option in the treatment of disease, but it is necessary to a better characterization of patients who could be benefit for theses therapies.19,20
We observed an important discordance between SCORAD, DLQI and SS. SCORAD evaluated three fields 2 objectives (area and intensity) and 1 subjective (symptoms of perception). DLQI and SS are totally subjective. The different purposes among scales could explain why most patients with good clinical control of pruritus reported a good control of DLQI and SS even with a moderate or severe SCORAD. This observation also suggests that pruritus plays a major role in the patient’s perception of control even more that eczema severity or the size of area affected. Some controlled studies have shown that antihistamines, have a poor effect in pruritus from patients with dermatitis,21 however, other studies indicate that may could help skin repair.22 The possible beneficial effects of antihistamines must be weighed against the known adverse effects such as sedation.23
One limitation of this study is that population included represented a convenience sampling and not all children with dermatitis from selected care centers were recruited. Nevertheless, the excluded children were similar in age and exposure to environmental conditions to the overall participants in TECCEMA cohort, although disease severity was greater in TECCEMA patients. Because dermatitis could be easily confused with other common skin diseases frequently present in the tropics (scabies, papules and others), it is possible that some of the children tested might have not dermatitis. Nevertheless, we included only patients with SCORAD > 8 with a diagnostic from an expert physician, so we believe that this possibility is unlikely.
In conclusion, dermatitis recommendations by the guidelines, allows achieving a significant reduction in the severity of eczema and improved quality of life. However, most patients in tropical region do not get complete control so it is necessary to develop better treatments account the special conditions of each region.











 nueva página del texto (beta)
nueva página del texto (beta)