Introduction
Status epilepticus (SE) has been defined as continuous seizures lasting equal or longer than 5 minutes, or ≥ 2 seizures without recovery to neurologic baseline in between seizures, as described by Lowenstein in 1999.1-7 It is clear that irreversible neurological damage occurs after 30 minutes of ongoing seizure activity, and several authors have acquired this timeframe to define SE.3,8-13 The incidence of SE in adults ranges from 17.1-60 patients per 100,000 annually in the United States, and accounts for 20% of all emergency room (ER) admissions for neurologic issues.1,3,4,14,15 It is more frequent in children, the elderly, and African-American individuals.1,12
When seizures cannot be controlled with first-line and second-line antiepileptic drugs (AED), despite the time elapsed from onset, it is defined as refractory SE (RSE).1,2,4,14,16 Some authors, such as Shorvon and coworkers, have taken into account the time, and consider SE refractory when there’s continuous seizure activity despite first and second-line anticonvulsant agents for ≥ 30-90 minutes, which has also been classified as «Stage 3» SE.3,17-19 During the third London-Innsbruck colloquium on SE in Oxford, the term «super refractory status epilepticus» (SRSE) was introduced, and it refers to the recurrence of seizure activity for ≥ 24 hrs despite treatment with general anesthesia, or, relapse of seizures when attempting to wean the patient off the anesthetic.17
Stages, classification and clinical features
SE has been divided into stages based upon its response to AED, and four stages have been proposed:2,3,17
Stage 1: Early SE-emergent/first line therapy Treat with first-line AED (benzodiazepines)
Stage 2: Established SE-urgent control/second line therapy Treat with second-line IV AED (fosphenytoin, phenytoin, valproate sodium, phenobarbital, midazolam, levetiracetam)
Stage 3: RSE Treat with general anesthesia (propofol, midazolam, pentobarbital)
Stage 4: SRSE Continue to treat with general anesthetics and consider alternative therapies (therapeutic hypothermia, ketamine, lacosamide)
SE can present as «generalized convulsive status epilepticus» (GCSE), focal motor SE (epilepsia partialis continua) or non-convulsive status epilepticus (NCSE).3,4,16 There is no universally accepted definition for NCSE yet, but it can be described as the SE that will not present with overt signs of convulsions but will display seizure activity in the electroencephalogram (EEG) and most commonly behavioral changes; it also frequently presents in the critically ill patient who remains comatose without a known cause.2,3,20 To date, there are 2 types of NCSE:
The «wandering confused» type, which consists of behavioral changes, inattentiveness, automatisms, facial twitching, nystagmus, delirium, among other signs; and
The critically ill patient with altered mental status (AMS), ranging from mild AMS to coma, with or without clinically subtle epileptic signs.2,3,16
Most GCSE will usually evolve into NCSE once it has «burn out» and it will be of great importance to differentiate it from de novo NCSE.16,20 This type of NCSE is better known as «end-stage SE» or «subtle SE» and it is characterized by coma with minimal or no overt convulsive activity anymore, and epileptic activity seen on the EEG, which is why it is advised to perform a continuous EEG in all patients presenting NCSE to monitor and prevent further complications.1,16,21,22
Etiology
One of the pillars of SE management and prevention of further complications is identifying and treating the underlying cause. Not all SE occur in patients with established chronic epilepsy, therefore, a thorough assessment should be done once SE is identified. The most common causes of SE are low levels of AED (in patients with known chronic epilepsy), accounting for 34% of the cases, abrupt discontinuation of AEDs, cerebrovascular accidents (CVA), metabolic imbalances (i.e. hypoglycemia, drug intoxications), systemic or central nervous system (CNS) infections or CNS tumors.2,3,16 Acute causes should be investigated and treated immediately, due to the greater danger this type of SE poses to the patient, and the higher risk of refractoriness it ensues.23
In comatose critically ill patients, the causes of seizures and SE may be different than those in outpatients.3 Hypoxemia and hypoxia account for 42% of the causes of SE in the ICU; stroke, drug substance toxicity, encephalitis, traumatic brain injury (TBI), vasculitis, CNS infection, and N-methyl-D-aspartate (NMDA) receptor antibodies are other causes to consider in this type of patients and should be treated promptly.3,16 The correct management of these entities will prevent the recurrence of seizures as well as a worse outcome secondary to complications and SE itself.12,16
Pathophysiology and diagnosis
The transition from self-limited seizures to SE is derived from the inability of the brain to control the exaggerated electrical activity by maintaining the balance between excitatory and inhibitory neurotransmitters, thus, failing to cease seizure activity and posing a danger to the patient.3,6,12 Gamma-aminobutyric acid (GABA) is the most common inhibitory neurotransmitter in the brain, which prevents excess neuronal excitation, and glutamate, which acts via NMDA receptor, the most common inhibitory one.6 The neuronal excitation resulting from the NMDA receptor stimulation occurs secondary to the entrance of calcium into the neuron and the subsequent depolarization; on the other hand, GABA inhibitory effects ensue thanks to chloride entrance into the post-synaptic cell, leading to hyperpolarization and excitatory antagonization.12 When a seizure ensues, there is an increase in excitatory activity and a decrease or failure of inhibitory mechanisms, however, this is usually self-resolving and the imbalance is rapidly corrected. A prolonged inability to restore the normal cerebral electrical activity with a sustained cell depolarization leads to the development of SE. The exact mechanism of how this is achieved remains unknown, but several theories have been proposed. One of the causative mechanisms of the development of SE is a shift in GABA and NMDA receptor function, called receptor trafficking.1 During prolonged seizures GABA receptors begin to decrease in number in the post-synaptic membrane, and eventually, receptor internalization takes place and receptors are degraded. This explains the consequent pharmacoresistance that presents in seizures lasting > 30 min, as seen in previous clinical studies, as well as the importance of controlling SE promptly.6,12,24-26 In contrast, NMDA receptors migrate to the synaptic membrane, enhancing neuronal excitotoxicity and resulting in irreversible neuronal damage.1),(3,6
According to the pathophysiological changes occurring at the time of presentation and the time elapsed from its onset SE can be divided into 2 stages: in stage 1, increased autonomic activity appears, and it presents with generalized tonic-clonic seizures, hyperpyrexia, hypertension and hyperglycemia.27 During the second stage, cerebral autoregulation is lost, and an increase in intracranial pressure (ICP) is seen; hypotension and decreased cerebral perfusion also appear.27 Patients enter stage 2 usually within 30 minutes after the onset of SE, and by then, most GCSE will have transitioned to subtle SE, stretching the importance of performing an EEG in comatose patients after overt convulsive activity has seemed to cease.21,27
Treatment
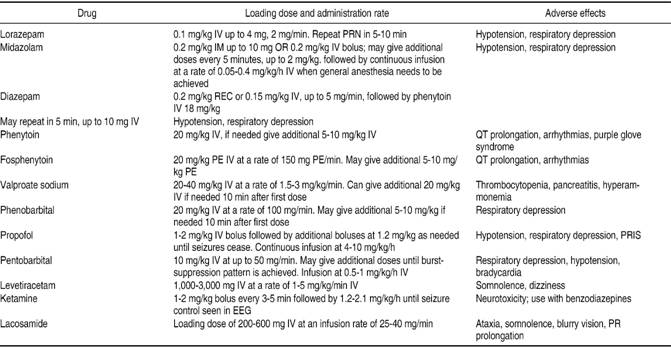
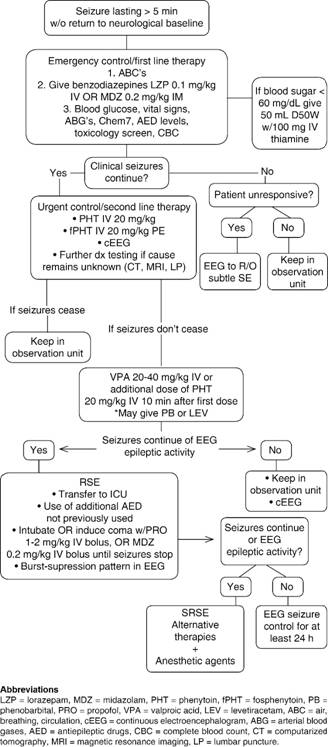
Prompt management of SE is the key to preventing pharmacoresistance and its consequent evolution into RSE or SRSE, as well as further complications; complete control of SE should be achieved in a timeframe of 60 minutes.28 Table I and Figure 1 depict the medications that can be used as well as a clinical algorithm that clinicians can use when confronted with a patient with SE.
The approach and treatment of SE is three-pronged and the main goals are:
There’s no available data on how many SE present in the out-of-hospital setting and how many in-hospital, so this review will discuss both pre-hospital and in-hospital initial management of SE.
Pre-hospital management
The management of SE in the pre-hospital setting is usually carried out by paramedics while transferring the patient to the nearest medical facility. Paramedics should assess and manage ABC’s (Airway, Breathing and Circulation) first and foremost. The airway should be protected, supplementary O2 given, and, if possible, IV access obtained.2 Finger-stick blood glucose should immediately be checked, vital signs obtained and a quick and targeted assessment made in the first 5 minutes. Benzodiazepines are the first-line agents to treat SE, whether it is in the pre-hospital or in-hospital setting. The PHTSE trial was a double-blinded, randomized study that evaluated the efficacy and safety of IV benzodiazepines as first-line treatment of SE compared to placebo in 205 patients.29,30 Of the 205 patients, 66 received lorazepam 2 mg IV, 68 diazepam 5 mg IV and the remaining 71 received placebo; a second dose was given if seizures reoccurred or did not respond to the first dose. On arrival at the ER, SE had terminated in 59.1% of patients treated with lorazepam and in 42.6% of the ones treated with diazepam; SE terminated in only 21.1% of those treated with placebo.30 The veterans administration (VA) cooperative study was a randomized, double-blinded, multicenter trial, of four IV AED regimens to treat SE in 518 patients.31 This trial found that lorazepam IV at a dose of 0.1 mg/kg terminated GCSE in 64.9% of patients, phenobarbital in 58.2%, diazepam followed by phenytoin in 55.8% and phenytoin alone in 43.6%.31 Although it was as equally effective as phenobarbital and diazepam followed by phenytoin, it is considered the drug of choice due to its rapid infusion, as opposed to the 20-minute infusion time needed with phenytoin.31 However, when there is overt GCSE it might be difficult to obtain IV access rapidly, therefore making IV administration of benzodiazepines less feasible. Also, the half-life of IV lorazepam is reduced when this is not refrigerated, diminishing its superiority and opening up the possibility of using an equally effective AED that can be administered through a more accessible route.24 In the RAMPART study, a double-blind, randomized clinical trial, the efficacy of IM midazolam vs. IV lorazepam in the pre-hospital management of SE was evaluated. Patients either received 10 mg of IM midazolam or 4 mg of IV lorazepam; IM midazolam was found to be equally as effective as IV lorazepam, terminating seizures in 73.4% of patients on arrival to the emergency department (ED), compared to 63.4% of those treated with IV lorazepam.32,33 Prasad and colleagues found, in a review of 18 studies with 2,755 participants, that midazolam IM might even be superior to lorazepam IV in the pre-hospital setting due to its lower hospitalization rate, increased efficacy in terminating seizures and decreased frequency of ICU admissions.34 The option of using an IM route in the pre-hospital management of SE decreases the length of time of AED administration, risk of pharmacoresistance due to the long time it takes to administer an IV AED and development of further complications.
In-hospital initial management and emergent/first line therapy
The emergent therapy begins with general measures, such as protection and preservation of the airway (intubation shall be done, if needed), assessment of vital signs and establishment of IV access; fingerstick blood glucose and arterial blood gases (ABG) should be immediately obtained since hypoglycemia, metabolic acidosis or hypoxia can be rapidly reversed and therefore, SE terminated.1,3 If the underlying cause of the SE is hypoglycemia, 50 mL of D50W together with 100 mg IV of thiamine should be given immediately.4 AED blood levels should be obtained as well as a complete blood count, liver function tests, urine toxicology screen, basic metabolic panel and magnesium, with the aim of finding the underlying cause of the seizures.1-3 Abortive treatment with first-line agents (benzodiazepines) should also be started promptly. Evidence supports IV lorazepam as the first drug of choice at a dose of 0.1 mg/kg; up to 4 mg of IV lorazepam can be administered and the clinician may repeat the dose within 10 minutes if epileptic activity isn’t aborted during this timeframe.1,2 If IV lorazepam isn’t available or cannot be given, IM administration of 0.2 mg/kg (up to 10 mg) of midazolam can also be considered, or rectal diazepam (20 mg).2,3 Respiratory depression and hypotension should be evaluated by the healthcare team, since these are common adverse effects of benzodiazepines.2,12
If seizure activity ceases, an anticonvulsant drug at a maintenance dose should be started to prevent the recurrence of seizures in all patients, however, if the underlying cause has been identified and treated, this measure may not be necessary.12,28
Urgent control/second line therapy
Urgent control therapy is needed when patients fail to respond to benzodiazepines (established SE) and seizure activity does not cease, and in those that have responded to first-line agents but require prevention of new onset of seizures.2 There is no sufficient data and evidence to determine which AED is superior for urgent control therapy, but current guidelines recommend the use of IV fosphenytoin/phenytoin, phenobarbital, valproate sodium, continuous infusion of midazolam and levetiracetam.2 By now, a continuous EEG should have been started in order to evaluate for possible subtle SE in patients that remain comatose after overt GCSE appears to have ceased.2 If the underlying cause remains unknown at this time, further diagnostic work-up should be initiated promptly. Brain imaging should be performed in order to evaluate for brain masses or lesions, and a lumbar puncture done to rule out CNS infection or an autoimmune cause.2 Uncommon etiologies, such as NMDA antibody or anti-voltage gated potassium channel antibodies should also be considered.16
Phenytoin and Fosphenytoin are FDA approved for the treatment of SE, and are the preferred agents for urgent control therapy.24 Phenytoin IV, is given as a loading dose of 20 mg/kg not exceeding 50 mg/min to avoid adverse effects and fosphenytoin IV 20 mg/kg given at a dosing rate of no more than 150 mg/min.12,24 For patients previously on phenytoin therapy, the loading dose should be modified in order not to reach serum toxic levels.12 Phenobarbital, is another FDA approved agent for the treatment of SE, however, it is not the most recommended AED since it acts in GABA receptors, making it a less feasible option for benzodiazepine-refractory SE.24 A meta-analysis by Yasiry and Shorvon demonstrated that the agent with the highest mean efficacy was valproic acid (75.7%), followed by phenobarbital (73.6%) and levetiracetam (68.5%); and contrary to what would be expected, phenytoin had an efficacy mean of roughly 50.8%.35 The established status epilepticus trial (ESETT), a multicenter, randomized, phase III trial, is currently the only randomized clinical trial aiming to determine which second-line AED is the most effective one in the setting of established SE.36 It will compare the effectiveness of fosphenytoin, levetiracetam and valproic acid in benzodiazepine-refractory SE in patients older than 2 years of age.36
Management of RSE
When clinical examination or EEG show persistence of epileptic activity despite the use of first-line and second-line AED, SE will be considered refractory and the use of additional agents or anesthetics will be necessary.16,37 Twelve to 43% of SE cases become refractory, and up to 60% of them occur in people with a previous history of epilepsy.4 In those patients without a previous history of seizures, acute brain injury or drug withdrawal will be the most common causes of RSE.16 Most cases of SE that respond to second-line agents will not be needing further intensive care, but those that remain comatose after GCSE and have failed to respond to urgent control treatment, will need to be transferred to an intensive care unit (ICU) for proper management.38
The pharmacological management of RSE involves either the use of additional second-line AED, or anesthetics at high enough doses to induce general anesthesia, with current guidelines recommending a continuous infusion of propofol, midazolam or pentobarbital.2 The decision of whether to use anesthetizing or non-anesthetizing agents for treatment of RSE remains subjective, and the clinician will have to consider etiology, type of SE, age and comorbidities to decide which agent to use.1,39
When coma needs to be induced, the patient has to be intubated, if not previously done, and continuous monitoring of vital signs will be mandatory.16 Midazolam IV can be given as a loading dose of 0.2 mg/kg over 2-5 minutes; boluses may be repeated every 5 minutes until seizures stop or a maximum loading dose of 2 mg/kg is reached.4 The loading dose will be followed by a continuous drip of 0.05-0.2 mg/kg/h.4,12 If breakthrough seizures present, a loading dose of midazolam will usually terminate the epileptic activity.12 One of the major advantages of midazolam is the lower incidence of hypotension, since this anesthetic doesn’t contain propylene glycol, as opposed to propofol.2 This last agent (propofol), may also be used to treat RSE as a loading dose of 1-2 mg/kg IV given over 3-5 minutes; repeat boluses, if needed, can be administered every 3-5 minutes and a maximum dose of 10 mg/kg should not be exceeded.4 Some of the advantages of propofol are its rapid onset and short half-life, which make it a convenient drug for tapering, and less tachyphylaxis than seen with midazolam, however, the development of hypotension requiring vasopressors in 50-70% of the cases, or the often fatal propofol infusion syndrome (PRIS) are major disadvantages of this drug.4,16,40,41 PRIS is a rare, but frequently fatal complication, of the use of prolonged infusion of the drug at high doses, and is characterized by arrhythmias, heart failure, hyperkalemia, hyperlipidemia, metabolic acidosis and rhabdomyolisis.4,17,40,41
Anesthetics should be titrated to induce a burst suppression pattern in the EEG when using propofol or barbiturates, and seizure suppression when using midazolam, for at least 24 hours.1,42
Another agent that has shown promise in the treatment of RSE is the noncompetitive NMDA receptor antagonist, ketamine.8 Synowiec and colleagues found, in a retrospective review, cessation of epileptic activity in all patients treated with ketamine (11 cases) in addition to other second-line AED, making it a plausible alternative option for treatment of RSE.8 NMDA receptor antagonists have also shown successful effects in terminating RSE in animal models, but human data is lacking, and further studies are needed in order to assess its efficacy in adults, and reach a consensus for the optimal dose to be used in this setting.8,43
Alternative therapies for SRSE
The term super-refractory SE (SRSE) is defined as an episode of SE that continues for 24 hours or more after the onset of administration of anesthetic agents or recurrence after the reduction of the same.44 An estimated 12-36% of patients with RSE develop SRSE, and the induction of a burst-suppression pattern becomes more difficult to achieve.15 When SE becomes super-refractory, experts recommend that AEDs be used synchronously with general anesthesia, and alternative therapies can then be implemented.35 Some of the new therapies that can be implemented during this stage of SE are ketamine, lacosamide, topiramate, mild therapeutic hypothermia, trigeminal nerve stimulation and transcranial magnetic stimulation.5,17,45-51 Hocker and colleagues suggest pentobarbital as the preferred barbiturate for treatment of SRSE. Pentobarbital is believed to be neuroprotective due to its effects on GABAA receptors and to lower core body temperature, which may also aid in the termination of epileptic activity.6
Therapeutic hypothermia (TH), widely used in the standard care of post-cardiac arrest patients, has recently started being used in the treatment of SRSE when all other measures fail.46 TH has been used by neurosurgeons during open surgery since the 1960’s due to its neuroprotective effects; it not only decreases the brain metabolism and intracranial pressure, but has effects on the sodium channels and reduces the activation of NMDA receptors, giving it a powerful anticonvulsive property.15,52,53
Varelas and colleagues recommend the use of mild TH (32-35 oC) for less than 48 hours in patients with SRSE, although Atkins and colleagues suggest that a more prolonged duration of cooling, as well as the use of an AED is necessary to achieve cessation of epileptic activity.3,54 Zeiler and associates performed a systematic review which included a total of 13 articles and 2 ongoing clinical trials, to investigate the effectiveness of TH as a therapy for RSE or SRSE.55 They retrospectively studied the cases of 40 patients (14 pediatrics, 18 adults and 8 of unknown age) with RSE and found that the addition of TH to AED’s achieved seizure control, defined as a burst-suppression pattern or seizure cessation in EEG, in 62.5% of the patients.55 Cooling blankets and endovascular devices were used for cooling, target temperatures ranged from 30-36 oC, the mean number of AEDs used previous to the start of TH ranged from 2-8 and the re-warming time varied in all studies.55 This systematic review has a grade 4 level of evidence and it was unknown whether TH alone or TH along with the AED’s was what finally controlled the epileptic activity, however, this systematic review gives scientists foundations to perform prospective multicenter trials to better study TH as a therapy for RSE and SRSE. In the authors’ own experience, the use of this therapeutic intervention is warranted. Presently, we use TH for 72 hours in patients with RSE and SRSE at a temperature of 32 oC.
As previously noted, ketamine, lacosamide and topiramate are also an option if not used before, to treat SRSE, along with other anesthetics or AED.56 A prospective observational study by Jaques and Rossetti evaluated the use of older vs. newer AED in the treatment of SE and their relationship with prognosis.57 Their results showed that the use of newer AED was related to poor prognosis, higher incidence of disability at hospital discharge, and had no correlation with mortality.57 Other therapies, such as magnesium infusion, ketogenic diet, and neurosurgical procedures are also currently studied, with no guidelines actually recommending them, and will not be further addressed in this review.56
Management of NCSE
An estimate of up to 25% of all cases of SE are the nonconvulsive type, and about 8% of comatose patients with no visible signs of seizures are experiencing NCSE.27
Intravenous benzodiazepines are the first line agents followed by phenytoin or fosphenytoin, but no AED has been chosen as a gold standard for NCSE yet. The treatment of recurrent electrgraphic nonconvulsive seizures (TRENdS) study was a prospective, multicenter, open-label, randomized study that aimed to compare the efficacy of Lacosamide versus fosphenytoin in NCSE presented in critically ill patients.58 The study was terminated in April 2014 and efficacy data is still being analyzed. Other studies suggest the early use of valproate if benzodiazepines fail to terminate the seizure.20 Yamamuro and associates reported a case of a 47 year old man that presented with NCSE with disinhibitory behavior, who was successfully treated with 1,000 mg/day of levetiracetam with few side-effects.59 Although the first clinical manifestation is altered state of consciousness accompanied by subtle motor twitches or behavioral changes, NCSE is commonly misdiagnosed leading to delays in treatment that affect the outcome and lead to the development of complications.20,60 A cEEG should be performed in any patient in whom NCSE is suspected, the cause promptly identified and addressed, and measurements to prevent further complications taken.
Prognosis
SE, if not properly managed, is associated with higher mortality ranging from 30-50%; an aggressive and accurate management of the underlying etiology and prevention of further complications are key to a favorable outcome.14,34,61 Cardiopulmonary complications and prolonged coma are factors associated with poor functional outcome.61 Prolonged ICU stay and complications related to prolonged mechanical ventilation were factors associated with higher mortality.61
Conclusions
SE is a true neurological emergency and clinicians should be able to recognize it with little to no delay in order to prevent irreversible neurological damage as well as complications that might emerge due to prolonged ICU stay, prolonged use of anesthetics in the setting of RSE, or adverse effects caused by AED.











 nueva página del texto (beta)
nueva página del texto (beta)