Study contribution
A failed involution of the navel stump in calves generates serious consequences for their health, as well as important economic losses. Traditional disinfection of this area with iodine solutions is marginally satisfactory. This study provides evidence of the almost-absolute efficacy of using enrofloxacin solvate and sodium-alginate gel to prevent navel infections in newborn calves, as well as to hasten navel involution/ detachment.
Introduction
The combined effects of deficient hygiene and late intake of colostrum can lead to a high incidence of navel illnesses.1 The navel in the newborn calf is probably the greatest health threat, as it can act as the gateway for bacteria to enter its body;2 yet swelling of the umbilical stalk, with or without herniation, is a common condition in calves.3 The most common pathogens encountered are Escherichia coli, Staphylococcus aureus, Streptococcus agalactiae, Pasteurella multocida, Proteus vulgaris, and Actinomyces pyogenes.4 It is known that the immediate intake of colostrum or at least during the first 2 h, can differentiate between an immunological competent calf and a less viable one.5,6
On most farms, shed space (farrowing area) is at a premium at calving time, and it is often undesirably humid, sometimes even more than other areas of the farm. Understandably, calves’ hygienic housing conditions are key to successfully handling calves’ navels. Moreover, it has been shown that clamping the navel is unnecessary and even unwise unless there is a hemorrhage, as this procedure favors infection.7 To prevent infections, navel dipping in an antiseptic solution has been recommended for the first two or three days of life as the stump dries out normally between 4 and 6 days. The disinfectants used include iodine, chlorhexidine, quaternary ammonium compounds, and other skin disinfectants. Navel dipping is a quick procedure, and it can reduce the risk of some serious diseases and even potentially the death of the calf.
If navel care is not meticulously performed, the calf´s temperament becomes dull and reluctant to suck and may stand with an arched back. Complications develop quickly causing sepsis. Some of the common signs of navel disease are fever, abscesses in the area and liver abscesses, septic arthritis, loss of appetite, and depression. If the infection is spread from the umbilicus into the peritoneal cavity, septic peritonitis develops and causes rapid deterioration in the calf’s condition. Abdominal distension is caused by gut stasis. The calf develops shock, and death occurs within 2-3 days.8 If the navel develops an infection, the treatment causes severe distress to calves, complications may require more veterinary care-time and may not always be successful.2,9
Most authors agree that apart from the hygienic measures and colostrum intake, navel dipping minimizes infections. However, despite the importance of umbilical cord care, very few randomized trials have been conducted to compare cord antiseptics, and the results may be surprising. Enrofloxacin HCl-2H2O (enro-C) is a new crystal-solvate of enrofloxacin (Patent MX/a/2013/014605; Instituto Mexicano de la Protección Industrial, Mexico City) with physicochemical and pharmacokinetic characteristics different from the standard enrofloxacin injection typically administered to calves for treating bacterial infections.10 Physicochemical properties of standard enrofloxacin have not facilitated its pharmaceutical preparation for topical use i.e. it dissolves at pH ≥ 10.4, and it is incompatible with calcium alginate. In contrast, the enro-C derivative is uniquely compatible with alginate, and its combination makes it possible to manufacture a therapeutic gel that releases enrofloxacin in a sustained manner and has already produced outstanding clinical results in canine pyoderma.11
Near Mexico City, there is a dairy farm industrial complex, intensively producing Holstein/Friesian cows. This production unit is located within the Agricultural and Industrial Complex of Tizayuca SA, Hidalgo, Mexico. It is important to point out that this place was chosen as the farms are all contained within an industrial complex of several dairies with high bovine population densities and poor hygiene and calves management approaches. Considering that an enro-C-alginate gel was available from the University of Mexico as experimental preparation, and that under the described scenario many navel illnesses were being presented, a clinical trial was carried out to compare the effects of the referred enro-C-alginate gel vs. the standard preventive treatment of navel dipping in a 10 % iodine-polyvinylpyrrolidone solution.
Materials and methods
Ethical statement
All study procedures and animal care activities were conducted in accordance with the Institutional Committee of Research, Care and Use of Experimental Animals of the National Autonomous University of Mexico (DC-2018-2-7).12
Animals and treatments
As previously stated, this trial was implemented at a dairy farm intensively producing Holstein/Friesian cows in the Agricultural and Industrial Complex of Tizayuca SA, Hidalgo, Mexico. A fairly good quantity of calvings per month in this farm allowed a total sample of 414 calves treated by navel dipping, either with 3 % enro-C as alginate gel (group: eCA, n = 209) or with an 11 % solution of polyvinylpyrrolidone iodine (group: I-PVP, n = 205) (Yodo Desinfectante®, Aranda Labs., México). A group treated with alginate alone was not regarded as ethical as preliminary tests resulted in navel infections and poor healing. This trial was carried out from January to May 2021.
The diagnosis of the likelihood of developing omphalitis was based on the local signs of inflammation and pain, swelling, raised local temperature, purulent or bloody discharge, thickening of the umbilical stump over 1.3 cm,13 shortness of the cord or navels ripped off or shortened due to dystocia and ill-handling of the newborn calf, the presence of an umbilical hernia, the need to clamp the stump due to hemorrhage were considered and of course, the degree of housing hygiene and overhandling of the navel when weighing the calf were also considered to design a risk level of developing a navel infectious process. The scoring algorithm of the enro-C group is presented in Table 1.
Table 1 Parameters to be considered in newborn calves (within 8 h after calving) to assign a risk level of developing a navel infectious process
| Risk | Navel length1 |
Navel width2 |
Respiratory edema3 |
Swollen tongue |
Hygiene in its environment |
Weakness in forelimbs |
Downer cow |
Calostrum intake (time and volume) |
| High | Absent or short | Wide | Evident respiratory distress | Marked | Poor | Cannot stand | Soon after calving | Later than 6 h and/or volume < 1 L |
| Medium | Medium | Thin | Slight respiratory distress | Slight | Medium | Stands with assistance | 4 h later | Later than 4 h and/or volume 1 - 2 L |
| Low | Long | Medium | No respiratory distress | Absent | fair | Normal wobble | 6 h later | Immediately after calving and up to 2 h and/or approx. 3 L |
1Classified as: absent; short, < 15.9 mm; medium, 56-85.9 mm; long, > 86 mm.
2Navel diameter classified as: thin, ≤ 10.9; medium, 11-22.9 mm; thick, ≥ 23 mm.
3A lot of liquid (marked and audible wet rales), discrete presence of liquid (barely audible wet rales); no liquid (no wet rales).
During the first 4 days, three independent and trained observers rated the involution of umbilical stumps as zero = no involution and/or having complications; 25 = slight involution; 50 = clearly visible involution; 75 = outstanding involution, and 100 = complete involution.
The calves were born in a specific area intended as a farrowing pen, floored with silica sand and straw on a dirt floor. Each umbilical cord was washed with running water to remove any particles from the silica sand, blood, and dirt. Then, they were immediately confined to individual calf-houses equipped with plastic roofs, a small area to move and sunbathe. The dirt floor was also bedded with silica sand and straw. Then, both treatments were generously smear-applied to the umbilical cords from top to bottom (usually less than 5 mL/umbilical stalk). This procedure was repeated once daily for four days.
The calves were then weighed and colostrum fed (2-4 L) at a temperature of 35-40 °C. Twelve hours later, calves were fed a second load of colostrum. During the physical examination, the cardiac and pulmonary functions were evaluated noting if marked-audible, slightly-audible or absent rales were present or not. Dexamethasone (0.02-1 mg/kg body weight), and bromhexine (0.5 mg/kg bw) were administered intravenously and intramuscularly, respectively, in the first two cases only. An umbilical plastic clamp was placed in few calves that presented navel hemorrhage. In these cases, it was removed on the third day.
Overall, calves treated with enro-C-alginate were 55 high-risk, 101 medium- risk, and 53 low-risk, while calves treated with I-PVP were 28 high-risk, 35 medium-risk, and 142 low-risk. The drying of the umbilical cord was assessed by daily observation and palpation, and on the fourth day, the endpoint was based solely on the presence or absence of the characteristic dry and thin aspect of the navel, with no tumors or fluid-bags in the calves’ abdominal wall. If inflammation, pain, heat, and/or discharge were present, the particular case was regarded as preventive-treatment failure, and the calf was assigned to an antibiotic treatment scheme for navel infection, out of this trial protocol. Additionally, all calves considered uninfected on day 4 were daily examined to determine the number of days taken for the dried-stump to detach, and the abdominal umbilical scar was examined.
Enro-C batches were prepared as indicated in Patent 472 715 (Mexico/Instituto Mexicano de Protección Industrial: IMPI MX/a/2013/014605 and PCT/Mx/ 2014/00192, Mexico City, Mexico). This process produces enrofloxacin hydrochloride-dihydrate with 99.97 % purity. The original molecule of enrofloxacin chemical-grade was purchased from Globe Chemicals (Mexico). The enro-C calcium alginate gel was manufactured with 2 % calcium alginate in distilled-sterile water containing 0.5 % propylene glycol and 3 % enro-C.
Statistical analysis
The statistical analysis for days to stump detachment was performed using mixed model methods (PROC MIXED; SAS software, version 9.1). Fixed effect interactions were also tested given the considerable sample size. Significance was set at P ≤ 0.05. Chi-square test was performed for the categorical variables and the statistical analysis was carried out using a categorical model procedure (PROC CATMOD; SAS software, version 9.1). All models included the fixed effects of risk level (high, medium, or low) and treatment.
Results
In the group treated with enro-C-alginate only one calf died. The necropsy diagnosis was peritonitis caused by hemorrhage due to the anatomical shape of the umbilical cord. Another calf from the high-risk group out of the 209 calves treated with enro-C-alginate as gel, developed umbilical cord fibrosis, but apparently without consequences for its normal growth and health. Two medium-risk calves underwent herniorrhaphy after treatment, and six presented with slight umbilical bleeding on day 4 (Table 2). In the group treated with I-PVP, 2 calves graded high-risk, 3 medium-risk, and 1 low-risk risk, out of the 205 developed cord fibrosis.
Table 2 Absolute frequency of clinical problems identified, days to stump detachment, and number of newborn calves (%) according to risk of developing an infection and complete navel involution after four days
| Group1 | Assessment of risk to develop an infection |
Complete navel involution on day 4 |
Clinical problems identified |
Mean ± SD days to stump detachment |
||
| High | Medium | Low | ||||
| Enro-C | 52/55 (94.5) |
97/101 (96.0) |
51/53 (96.2) |
200/209 (95.7) |
1. fibrosis 2. herniorrhaphy* 6 umbilical bleedings |
29.7 ± 0.8 |
| I-PVP | 22/28 (78.6) |
28/35 (80.0) |
111/142 (78.2) |
161/205 calves (78.5) |
44 omphalitis and omphalophlebitis 1 umbilical bleeding |
32.9 ± 3.1 |
1Enro-C: enrofloxacin hydrochloride-dihydrate and calcium alginate gel; I-PVP: 10 % solution of polyvinylpyrrolidone iodine.
Clinical problems identified and days to stump detachment denote statistically significant differences. Chi-square test was performed (χ 2 = 61.047; P < 0.0001).
*Deemed necessary as hernia sac/opening was more than six cm wide.
Forty-four calves developed cord infection: 13 from the high-risk group, 11 from the medium-risk group, and 20 from the low-risk group. Only a calf from the medium-risk group showed umbilical bleeding on the fourth day.
Regarding the pace of the umbilical cord involution, it stands out that at 24 h most stumps were noticeably dried in calves from the enro-C group. Their stump was detached within an average of 30 days (Table 2) and the umbilical scar did not present infection. Conversely, calves treated with I-PVP and those that did not develop an infection had similar degree of stump drying after 72 h, and stumps detached within an average of almost 33 days. The difference between the groups was statistically significant in favor of the enro-C group at all three risk levels (P < 0.05 in all cases).
In Figure 1, the progression of a navel treated with enro-C-alginate is presented in 3 images. Figure 2 shows the same progression in non-infected calves, treated with I-PVP. Figure 3 shows the cumulative percentage of umbilical stump involution for both groups with a CI = 95 %. It was noted that with the enro-C alginate the sand was less sticky to the stump. The enro-C alginate gel formed a layer around the umbilical scar in calves that did not have a cord or that had a very short cord. This layer prevented the accumulation of dirt in the hole. In contrast, the I-PVP dried relatively quickly and did not form a layer similar to that described around the stump and/or the umbilical scar. Additionally, it was observed that with the I-PVP the cords became dirtier. Multiple comparisons were carried out by Tukey-t test.
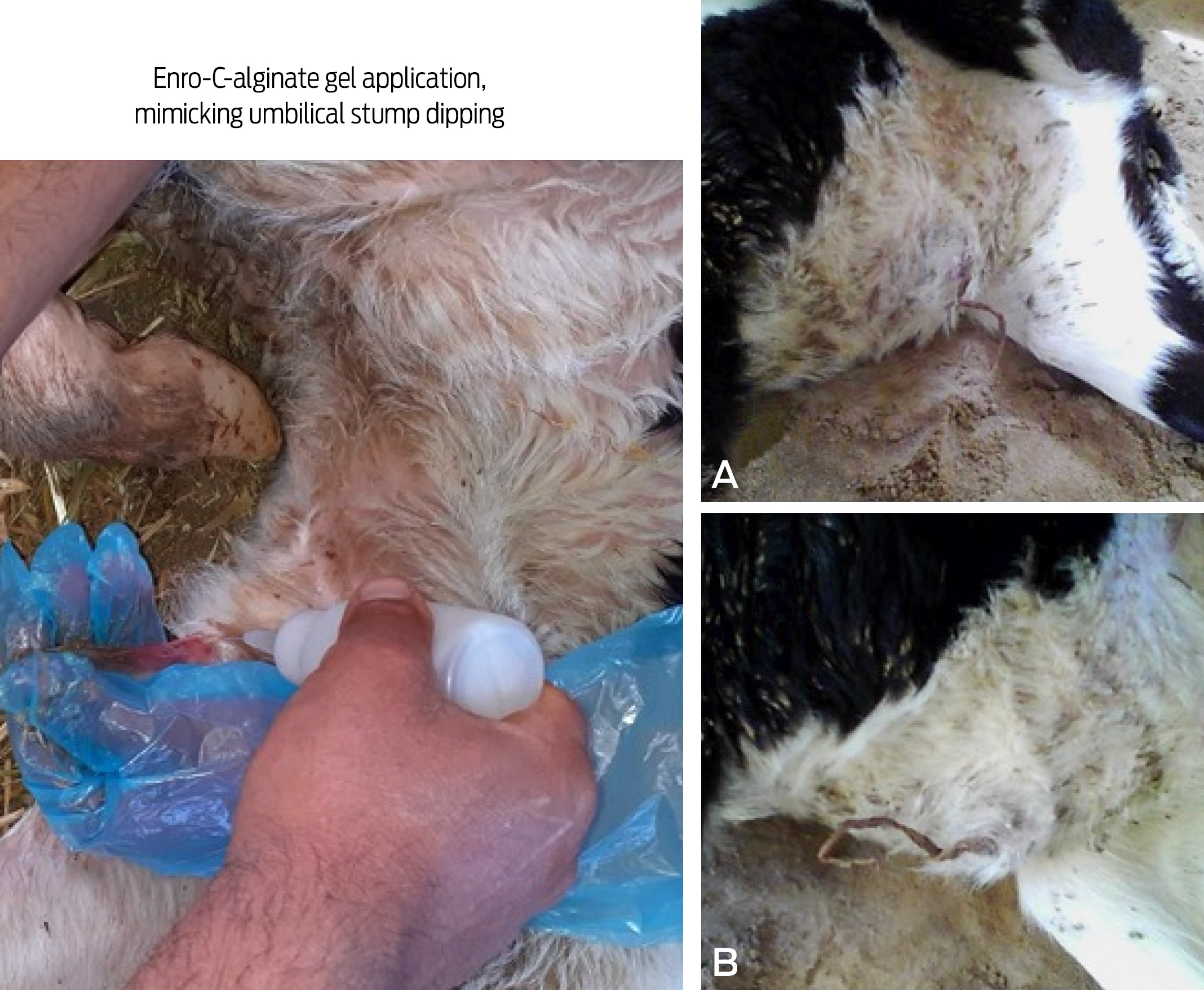
Figure 1 The enro-C-alginate gel being applied in a calf’s umbilical cord (left). Shape and degree of involution of the umbilical stump at 24 h (A), and 48 h (B), showing it was almost completely dried/involuted.

Figure 2 Images of the iodine-polyvinylpyrrolidone (I-PVP) being applied in a calf’s umbilical cord. (A) and (B) panels show incomplete involution of the umbilical stump at 24 h, and 48 h, respectively.
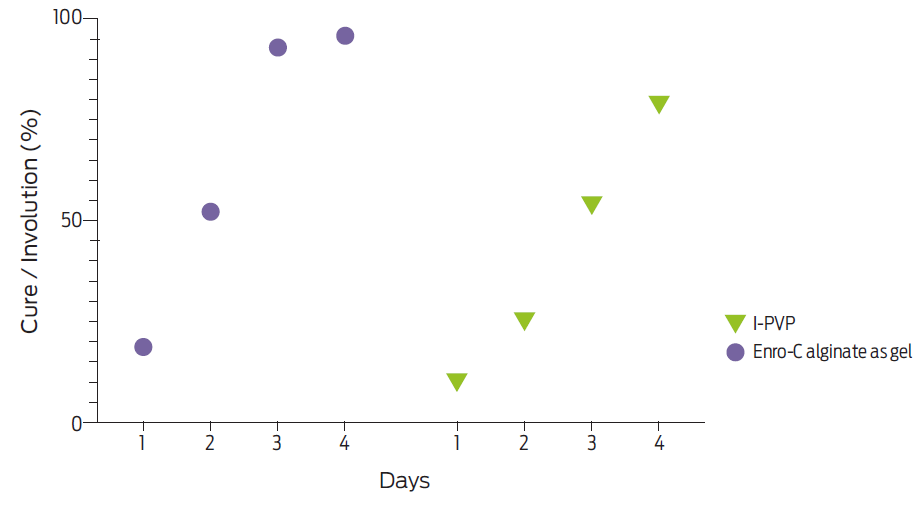
Figure 3 Per cent cure/involution of umbilical stumps in calves treated for three consecutive days with either enrofloxacinealginate gel (“Enro-C alginate as gel” group, n = 209), or iodine-polyvinylpyrrolidone (I-PVP group, n = 205). Differences in involution initiated at 2.3 % and were 43.9 % different on day 4.
Discussion
It has been suggested that between 5 and 20 % of dairy calves in the United States develop umbilical infections,14,15 and 1.6 % of reported calf deaths are related to umbilical infections.16 Hence, efforts have been made to find the most suitable preventive treatment and handling procedures to have a prompt and uneventful navel detachment.17-20 For example, Robinson et al.8 compared, in newborn Jersey calves, four disinfectants: 7 % iodine tincture, 0.1 % chlorine with an alleged new technology, 4 % chlorhexidine gluconate, and 10 % trisodium citrate. No statistically significant advantages were found among them.
In another study, chlorhexidine was statistically superior to iodine and it was found that iodine tended to increase mortality risk.21 Fordyce et al.22 also challenged four preventive treatments: 7 % iodine, a dry navel bath based on nisin (a bacteriocin produced by a group of Gram-positive bacteria that belongs to Lactococcus and Streptococcus species) mixed with talcum powder (3.105 g nisin per 100 g of talcum powder), liquid nisin at a concentration of 64 µg/mL, and 4 % chlorhexidine in alcohol (1:1). Again, results revealed no statistically significant differences among these treatments. However, these authors concluded that the diameter of the umbilical cord at birth significantly influenced the resolution of the umbilical scar when quantified within the first 48 h after birth. This parameter loss its positive correlation with a proper involution of the umbilical stump when quantified after 48 h.
In this study, a much better resolution of the umbilical scar was obtained using enro-C than with I-PVP, both in the number of infection-free calves on day 4 and in the speed of stump detachment (P < 0.05 in both cases), while for enro-C, no differences could be detected depending on the risk assessment classification. However, apart from the studies reported by Fordyce et al.22 in which nisin is used, there are no reports on the use of antibiotics to handle the umbilical stump immediately after calving, and only skin antiseptics are recommended. It is therefore important to test whether the bacterial resistance rate to this drug and other fluoroquinolones, is increased by the use of enro-C in the routine preventive treatment of umbilical stumps.
Alginate products have been proposed for wound healing long ago.23 In particular, calcium alginate is a highly absorbent, biodegradable dressing derived from seaweed and as a non-woven dressing has shown to have positive effects in wound healing.24 Moreover, alginate-based hydrogel films have been successfully used for wound healing.25
Early studies showed that the formation of the scar and the rate of epithelisation of superficial wounds was accelerated.26 Hence, many alginate-based wound dressings are commercially available,27 as it has been shown that this material retains bacteria within it and avoids their spreading and growth,28 while keeping the affected area isolated and allowing oxygen diffusion.29 Furthermore, improvements to the alginate matrix have been done. For example, tributylammonium alginate fibrous mat accelerates wound healing of full-thickness skin wounds.30 However, although other drugs have been incorporated to achieve a sustained release,31 to the best of our knowledge this is the first time an alginate gel including an antibacterial drug has been successfully prepared and tested in a clinical setting.
In particular, the enro-C solvate crystal we used is physicochemically compatible with calcium alginate to form a gel, and many other antibacterial drugs tested were not suitable to produce a stable drug formulation.
In previous laboratory control studies in rats (not shown), calcium-alginate as gel showed a noticeably weak healing effect as compared to the enro-C-alginate gel preparation. Although not considered within the scope of this trial, it is worth mentioning that it is tempting to propose that the enro-C/calcium alginate hydrogel obtained undergoes a unique structural modification, resulting in a matrix with Ca ions attached that allows a sustained release of enro-C.32 Wound dressings and in this case, navel stump protection is likely to keep progressing. Further research is needed to determine whether enro-C under different clinical scenarios ensures a proper involution and detachment of the umbilical stump in calves.
Conclusions
This study showed enro-C-alginate gel is a more effective alternative to prevent calves’ navel infections compared to I-PVP. The enro-C treatment also promoted the rapid and successful involution of the umbilical scar and the detachment of the umbilical stump. It is important to assess enro-C performance in other scenarios. Moreover, further studies are required to assess the impact of enro-C treatment would have on the development of fluoroquinolone resistance in the farm.
Data availability
All relevant data of this research are presented in the paper. The datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Conflicts of interest
All authors declare that they have no conflicts of interest concerning this publication.
Author contributions
Conceptualization: L Gutierrez, H Sumano.
Formal analysis: G Tapia-Perez.
Investigation: M Rodriguez, E Posadas, H Sumano.
Methodology: D Dominguez, M Amigon.
Supervision: M Rodriguez, N Rodriguez.
Writing-original draft: L Gutierrez, H Sumano.
Writing-review and editing: L Gutierrez, H Sumano.











 nueva página del texto (beta)
nueva página del texto (beta)



