Study contribution
Leptospirosis is a bacterial zoonosis affecting humans and numerous animal species and is frequently classified as a "neglected disease" due to its high underdiagnosis. Domestic cats can be infected with leptospires, but their role in the disease transmission and the maintenance of the bacteria in the environment is not clear. This systematic review and metaanalysis state the global prevalence of leptospirosis in domestic felines analyzing published studies using indirect diagnostic tests (detection of antibodies) and direct diagnostic tests (recognition of the bacteria in urine and/or kidney samples) according to the continent and origin of the animals. Risk factors for the presentation of the disease are also described, as well as the most reported serovars and serogroups. this information contributes to a global vision of the frequency of presentation of the disease in domestic cats, which is relevant, considering that felines are very popular pets, and their population is increasing worldwide.
Introduction
Keeping companion animals and the close relationship between humans and pets can increase the risk of zoonotic disease transmission. For this reason, epidemiologic studies aimed at determining the frequency of presentation and prevalence of zoonoses in animal populations are of great relevance for public health1. Domestic cats can be reservoirs of different infectious agents, posing a risk of disease to other cats, wildlife species, domestic animals and humans2 and their role in diseases such as toxoplasmosis, catscratch disease, plague, larva migrans syndrome and rabies has been well documented.3
Cats are incidental hosts of a variety of serovars of Leptospira prevalent in different animals.4 Outdoors animals have an increased risk of becoming infected since they are in close contact with maintenance hosts and felines living in rural areas can also become infected from cattle and swine contact.5 Some studies have established the renal carriage of Leptospira species by PCR in cats, confirming that felines could be a chronic reservoir host for the bacteria and a possible risk factor for human infection.56 In one study, leptospiral DNA was detected in renal tissue, urine, and blood respectively in 14.3 %, 10.3 % and 11.9 % of stray cats.7
Leptospira infection in cats is not always associated with clinical signs and limited information about the diagnostic test performance and treatment options is available8, 11 Consequently, the understanding of the epidemiology of the disease is poor and the relevance of domestic felines in the zoonotic transmission of the disease is not totally understood.12, 14 This is a concern because leptospirosis is recognized as a public health problem especially in developing countries,15 and the disease is considered a good example of the "One Health" approach because intra- and interspecies transmission is dependent on the reservoir host animals in which the bacteria replicate and are shed in urine, as well as the persistence of the bacteria in the environment, and the subsequent human animal environmental interaction.16 This approach is essential because in Leptospira, human infection invariably results either from direct animal exposure or from contact to environments contaminated by infected animals.17 The humananimalenvironment interaction in Leptospira infection can help to increase the knowledge about the emergence of new cases, which is a major challenge.18, 19
Metaanalysis combines the findings of different studies into single pooled results; is a powerful tool of synthesis for scientific research.20 Some metaanalyses comprising epidemiologic aspects of leptospirosis in domestic dogs have been published21, 22 but to date no systematic review or metaanalysis in domestic cats has been reported. Thus, the aims of this study were: 1) to perform a systematic review to describe epidemiologic aspects of published studies on leptospirosis in cats, focusing on geographic location (continent), decade of publication, reported serovars and serogroups of Leptospira, factors associated with the disease and origins of the sampled cats and, 2) obtain pooled measures of the global prevalence of the disease in cats with a meta-analysis of studies using indirect diagnostic tests (detection of antibodies) (M1) and another with studies using direct diagnostic tests (detection of the bacteria in urine and/or tissue samples) (M2).
Materials and methods
Bibliographic search strategy:
To find observational epidemiologic studies reporting the prevalence of leptospirosis in domestic cats, a bibliographic search was conducted in scientific electronic databases (EbscoHost, Science direct, Springer link, Willey InterScience, Pubmed, Redalyc and Scielo), and in search engines (Google and Google Scholar). Documents in full text and abstracts, original articles, short communications, thesis, conference presentations and letters to the editors were considered.
The documents searched were published between January 1920 and October 2020. The key words used were leptospirosis cats, leptospira cats, feline leptospirosis, prevalence (English language), leptospirosis gatos, leptospirosis felinos, prevalencia (Spanish language) and leptospirose gatos, prevalência (Portuguese language). These key words were combined to perform a comprehensive bibliographic search. For example, the combinations used were: "leptospirosis cat prevalence", "Leptospira cat prevalence", "feline leptospirosis prevalence" in English as in the other languages.
Eligibility criteria of the studies:
Inclusion criteria:
Observational epidemiologic studies using indirect diagnostic tests (serology, such as MAT, Latex Agglutination Test, Rapid Slide Agglutination Test, ELISA) and direct diagnostic tests (for example: PCR, bacteriologic culture, and others) with clearly defined diagnostic criteria for considering the samples as "positives" or "negatives" to pathogenic Leptospira were considered. Only full texts and abstracts with a clear report of the prevalence of leptospirosis and / or documents indicating the sample size and the number of positive animals for the diagnostic test used were selected, as well as studies of evaluation of diagnostic tests if they reported the prevalence, sample size and number of positive animals.
Exclusion criteria:
Literature reviews, case reports, clinical trials and duplicated articles were omitted.
Methodologic quality evaluation of the studies:
The methodologic quality of the studies was evaluated using some features described by O'Connor et al.23 in the veterinary extension of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) such as the study design, aims of the study, geographic location, diagnostic test used, diagnostic criteria, sample size and main results. When a document fulfilled the selected characteristics, it was considered as having acceptable methodologic quality. The criteria for documents in full text and abstracts are detailed in Table 1.
Table 1 Characteristics of studies in full text and abstracts considered in the evaluation of their methodologic quality
| Characteristics considered in full text studies: |
| 1. The study design was observational. |
| 2. The objective(s) of the study is / are stated. |
| 3. The geographic location in which the study was carried out, relevant dates, including periods of recruitment and data collection are described. |
| 4. The document indicates the eligibility criteria for the owners / managers and for the animals, the sources, and methods of selection for the owners and for the animals and the method of followup (if it is applicable). |
| 5. The study clearly defines the diagnostic criteria. |
| 6. The document defines the diagnostic test used and the sample size. The main results are described (prevalence), or the number of positive animals, or unadjusted estimates and their precision (for example 95 % Confidence Intervals). |
| Characteristics considered in the abstracts: |
| 1. The title and the purpose of the abstract allow for the identification of the research topic and the general design of the study. |
| 2. The abstract indicates that the study design was observational. |
| 3. The objective(s) of the study is / are stated. |
| 4. The document indicates the geographical location where the study was performed. |
| 5. The document defines the diagnostic test used and the sample size. The main results are described (prevalence), or the number of positive animals, or unadjusted estimates and their precision (for example 95 % Confidence Intervals). |
Data extraction:
The data extracted in each selected publication were: 1) author(s), 2) year of publication, 3) materials and methods: study design, diagnostic test used (indirect and/or direct diagnostic test), sample size, geographic location, 4) origin of the sampled cats, 5) results: number of positive animals in the diagnostic method, serological information (serovars/serogroups included, if MAT was used as diagnostic test), prevalence (if it was reported. If not, the number of positive animals and the sample size was recorded), risk or protection factors potentially related to leptospirosis with the measure of risk (for example: odds ratio, relative risk), confidence intervals (95 % CI) and/or P-values associated with the measure of risk. In studies with no prevalence informed, it was calculated using the formulae (modified from Dohoo:24 Prevalence = positive animals in the diagnostic test/study sample size.
Statistical analysis:
For the systematic review, the most frequent geographic locations (continent), decades of publication of the studies, Leptospira serogroups and serovars, risk or protection factors associated with the disease were determined, as well as the origin of the sampled cats (for example: patients in veterinary hospitals, owned cats, stray or feral cats).
The selected studies were classified into two groups to conduct a meta-analysis in each one to obtain a pooled measure of the prevalence: 1) studies using indirect diagnostic test or serology (M1), and 2) studies using a direct diagnostic test (M2). The statistical heterogeneity betweenstudies was assessed with the Q statistic Test (P < 0.1):25 complemented by the inconsistency test (I2), which indicates percentages of heterogeneity (a value greater than 50 % was considered as indicator of heterogeneity). The Tau2 (T2) was also calculated as a quantification of the betweenstudy variance:26 considering a value > 0.27 The random effects model was used to obtain the pooled approximation of the prevalence because it is more likely to fit the sampling distribution and it does not consider a restriction of a common effect size.28 Forest plots were created to illustrate the prevalence of each study, as well as the pooled estimation.
The Begg and Egger tests (P < 0.1) were conducted for publication bias detection. A subgroup analysis was done to explore potential sources of heterogeneity.25, 26, 29 The continent, origin of the sampled cats and some factors associated with the disease were used as subgroups. Meta-analyses were performed with MIX Pro version 2.0.30 The measure of effect was the prevalence, and the Freeman Tukey double arcsine transformation was used to obtain the standard error. The Inverse variance (Ivt) with T2 method of weighing for random effects model analyses (onestep Der Simonian and Laird method) was executed. The PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses)31 was followed to write this study.
Results
Systematic review:
Bibliographic search and evaluation of the methodologic quality of the studies:
In the bibliographic search, 139 articles about Leptospira or leptospirosis in domestic cats were found. The studies were published between 1938 and 2020. Out of 139, 75 (53.96 %) were original articles, 42 (30.22 %) were abstracts, 5 (3.6 %) were theses, 5 (3.6 %) were letters to the editor, 4 (2.88) were short communications, 4 (2.88 %) were conference presentations, one article (0.72 %) was a case report and in 3 (2.16 %) documents the type of study was not described. Out of the total number of retrieved articles, 46 were eliminated. Finally, 93 studies were considered for a full review and methodologic quality evaluation. Two databases were created: one for studies that used indirect diagnostic tests and other for those that based their results on a direct diagnostic test.
The database of studies with indirect diagnostic tests included 83 documents and the diagnostic tests involved MAT (n = 80 studies), ELISA (n = 2), Latex Agglutination Test (n = 1) and Rapid Slide Agglutination Test (n = 1). In the database of studies with direct diagnostic tests, 23 articles were incorporated. The tests included were PCR (n = 19 studies), bacteriologic culture (n = 3), PCR/bacteriologic culture (n = 1) and Loop Mediated Isothermal Amplification (n = 1). All the studies subjected to methodologic evaluation has an acceptable methodological quality, and it were included in the meta-analyzes. The flow chart in Figure 1 describes the process of bibliographic search and selection of articles.
Epidemiologic aspects of published studies:
Geographic location:
South America, Europe and Asia were the most frequent geographic locations of the articles (Table 2).
Table 2 Continents of the studies included in the systematic review
| Indirect diagnostic tests | Direct diagnostic tests | ||||
|---|---|---|---|---|---|
| Continent | Number | % | Number | % | |
| North America | 10 | 12 | 2 | 8.7 | |
| Central America | 4 | 4.8 | 1 | 4.3 | |
| South America | 25 | 30.1 | 3 | 13 | |
| Europe | 21 | 25.3 | 6 | 26.1 | |
| Asia | 14 | 16.9 | 8 | 34.8 | |
| Africa | 0 | 0 | 1 | 4.3 | |
| Oceania | 5 | 6 | 1 | 4.3 | |
| AsiaAfrica | 2 | 2.4 | 1 | 4.3 | |
| No information | 2 | 2.4 | 0 | 0 | |
| Total | 83 | 100 | 23 | 100 | |
M1 (meta-analysis of studies using indirect diagnostic tests) and M2 (meta-analysis of studies using direct diagnostic tests).
Decade of publication:
Documents published between decades of 1940 and year 2020 using indirect diagnostic tests were found, with more frequency of publication in the decades 2010 and 2000. For articles using direct diagnostic tests, documents published in the decades of 1970, 2010 and in the year 2020 were found, but mainly published in the 2010 decade (Table 3).
Table 3 Decades of publication of the studies included in this review
| Indirect diagnostic tests | Direct diagnostic tests | ||||
|---|---|---|---|---|---|
| Decades | Number | % | Number | % | |
| 1940s | 1 | 1.2 | 0 | 0 | |
| 1950s | 2 | 2.4 | 0 | 0 | |
| 1960s | 2 | 2.4 | 0 | 0 | |
| 1970s | 7 | 8.4 | 2 | 8.7 | |
| 1980s | 7 | 8.4 | 0 | 0 | |
| 1990s | 6 | 7.2 | 0 | 0 | |
| 2000s | 11 | 13.3 | 0 | 0 | |
| 2010s | 40 | 48.2 | 18 | 78.3 | |
| Year 2020 | 7 | 8.4 | 3 | 13 | |
| Total | 83 | 100 | 23 | 100 | |
Serovars and serogroups of Leptospira in studies using indirect diagnostic tests (MAT):
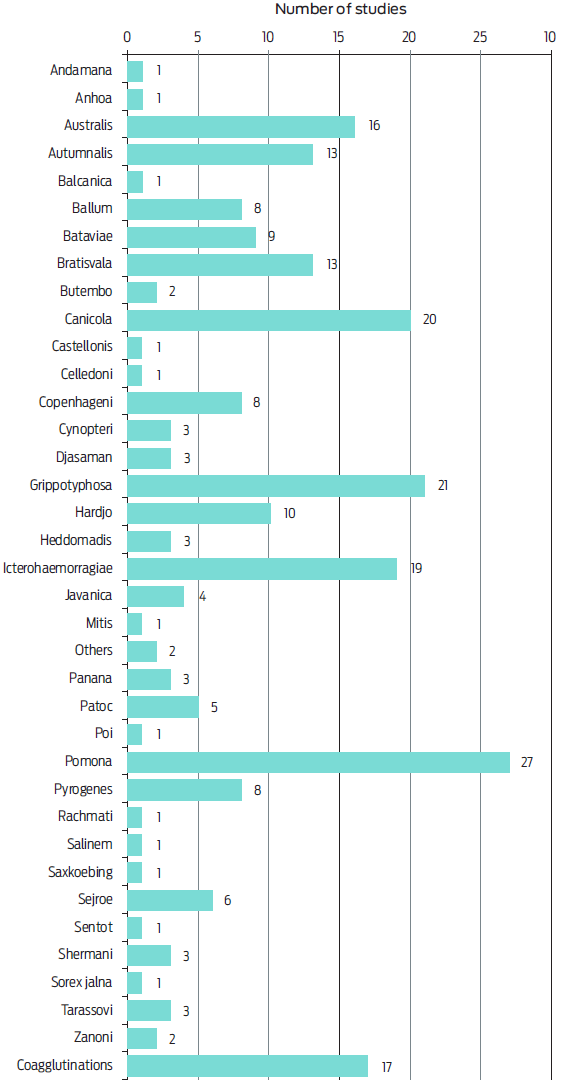
The serogroups of Leptospira causing serologic reactions were recorded in 16 articles. Seropositivity to 19 different serogroups was found and the most frequent were Icterohaemorragiae (n = 9 studies), Australis, Autumnalis and Pomona (n = 7 studies) (Figure 2). In 59 studies, seropositivity to 36 different serovars was noted, as well as different coagglutinations. The most common reactions were for serovars Pomona (n = 27 articles), Grippotyphosa (n = 21), Canicola (n = 20) and Icterohaemorragiae (n = 19) (Figure 3).
Factors associated with leptospirosis:
Eleven studies described factors associated with the disease. These were published in the decades of 2000, 2010 and in the year 2020, carried out in North America, South America, Europe, and Asia (Table 4).
Table 4 Factors associated with leptospirosis in domestic cats described in different studies
| Study | Country | Factors associated with leptospirosis | OR | 95 %CI | P-value |
|---|---|---|---|---|---|
| Azócar-Aedo et al.32 | Chile | Activities with water that flows in streams or backwater | 38 | 1.9-763.9 | … |
| Habitat near flooded areas | 44.5 | 1.4-145.5 | … | ||
| Dorsh et al.6 | Chile | Health status: sick | 3.04 | 1.1-8.39 | … |
| Previous vaccinations | 2.93 | 1.18-7.24 | … | ||
| Letha et al.33 | Estonia | Pet cat with access outdoors | 7.45 | 2.51-22.12 | < 0.001 |
| Shelter cat | 9.98 | 3.15-31.57 | < 0.001 | ||
| Eastern Estonia | 0.36 | 0.16-0.81 | 0.013 | ||
| Longhurts34 | United Kingdom | Lymphocytes count | 1.42 | … | 0.029 |
| Basophils count | 4.62 | … | 0.032 | ||
| Age versus acute kidney injury | 0.83 | … | 0.002 | ||
| Moreira da Silva et al.35 | Portugal | FIV | 0.35 | 0.14-0.85 | 0.02 |
| Murillo et al.36 | Spain | Without statistically significant factors | NA† | NA | NA |
| Ortega-Pacheco et al.37 | Mexico | Access to street | 3.2 | 1.8-8.3 | 0.01 |
| Parreira et al.38 | Brazil | Age and urea | 2.82 | 1.48-5.39 | 0.002 |
| Age and alkaline phosphatase | 3.42 | 1.81-6.47 | 0.001 | ||
| Age and creatinine | 0.34 | 0.18-0.64 | 0.001 | ||
| Rodriguez et al.39 | Canada | Kidney disease | 2.8 | 1.2-6.6 | 0.02 |
| Known hunter (yes) | 3.4 | 1.4-8.3 | < 0.001 |
Forty-four factors associated with leptospirosis were found. Out of these, 21 were statistically significant (Table 4). The variables were related with lifestyle, age, maintenance conditions and biochemical parameters. Seventeen variables were risk factors and four were protective factors. Two variables coincided among publications: 1) "access to outdoors or street" in the studies by Letha et al. (20 20)33 (OR = 7.45; 95 % CI = 2.51-2.12) and Ortega-Pacheco et al. (2020)37 (OR = 3.2; 95 %CI = 1.8-8.3) and, 2) "hunter/hunting rodent habit" in the articles by Rodriguez et al. (2014)39 (OR = 3.4; 95 %CI = 1.4-8.3) and Rose et al. (2020)40 (OR = 8.9).
Pooled measures of the global prevalence of leptospirosis in cats: metaanalyses of studies using indirect and direct diagnostic tests
Metaanalysis of studies using indirect diagnostic tests (M1):
A global prevalence of 11.09 % (95 % CI = 8.68-13.73) was calculated. The Q test (P-value = 0), the I2 test (91.07 %; 95 % CI = 89.39-92.49 %) and T2 estimation (0.0198; 95 %CI = 0.0164-0.024) indicated heterogeneity.
No evidence of publication bias was indicated by the Begg test (P = 0.20), in contrast with the Egger Test (P = 0.009).
Metaanalysis using direct diagnostic tests (M2):
A pooled prevalence of 9.22 % (95 % CI = 4.30-15.41) was estimated. Heterogeneity was detected (Q test P-value = 0; I2 test = 92.86 %; 95 % CI = 90.33-94.74 %; T2 estimation = 0.0327; 95 % CI = 0.02349-0.04529).
The Begg (P = 0.03) and the Egger tests (P = 0.02) indicated publication bias.
Subgroup analysis:
In the subgroup analysis for studies using indirect diagnostic tests, the highest pooled prevalences were observed in Oceania (11.94 %; 95 % CI = 9.27-14.86) and Europe (10.35 %; 95 % CI = 9.39-11.35), while the lowest was recorded in Central America (6.90 %; 95 % CI = 3.55-11.11). In articles with direct diagnostic tests, a higher prevalence was observed in Asia (18.14 %; 95 % CI = 5.41-35.41) and the lowest was noted in North America (1.6 %; 95 % CI = 0.025-4.76) (Table 5).
Table 5 Subgroup analysis results
| Indirect diagnostic tests | Direct diagnostic tests | |||
|---|---|---|---|---|
| Subgroup analysis: | Pooled prevalence | 95 % CI | Pooled prevalence | 95 % CI |
| 1. Continent: | ||||
| North America | 8.69 | 6.9-10.63 | 1.6 | 0.025-4.76 |
| Central America | 6.9 | 3.55-11.1 1 | NA† | NA |
| South America | 7.28 | 5.86-8.82 | 5.79 | 0.025-17.94 |
| Europe | 10.35 | 9.39-11.35 | 4.62 | 1.2-9.67 |
| Asia | 8.72 | 7.12-10.45 | 18.14 | 5.41-35.41 |
| Asia-Africa | … | … | NA | NA |
| Africa | NA | NA | NA | NA |
| Oceania | 11.94 | 9.27-14.86 | NA | NA |
| 2. Some origins of the sampled cats: | ||||
| Different origins | 6.32 | 4.54-8.36 | NA | NA |
| Free roaming | 9.03 | 2.42-18.4 | NA | NA |
| Veterinary hospital patients | 11.22 | 6.32-17.16 | 12.72 | 7.15-19.55 |
| Household and stray | 10.88 | 3.31-20.96 | NA | NA |
| Neuter campaign | 11.08 | 3.7-21.59 | 1.54 | 0.31-3.43 |
| Outdoor cats | 14.25 | 7.66-2.41 | NA | NA |
| Owned cats | 8.67 | 3.86-14.9 | 1.36 | 0-4.38 |
| Rural | 14.55 | 2.56-33.19 | NA | NA |
| Shelter | 16.48 | 10.84-22.98 | NA | NA |
| Stray | 3.6 | 0-14.11 | 17.21 | 7.1 1-30.24 |
| Feral | NA | NA | 8.24 | 0-34.2 |
| 3) Factors associated with leptospirosis: | ||||
| Access to outdoors or street | 14.9 | 10.45-19.99 | NA | NA |
| Hunter / hunting rodent habit | 11.38 | 4.24-21.24 | NA | NA |
†NA = Not applicable
Regarding the origins of some of the sampled cats, with indirect diagnostic tests, higher pooled prevalences were noted in shelter (16.48 %; 95 % CI = 10.84-22.98), rural (14.55 %; 95 % CI = 2.56-33.19) and outdoor cats (14.25 %; 95 % CI = 7.66-22.41). In studies using direct tests, elevated pooled prevalences were assessed in stray cats (17.21 %; 95 % CI = 7.11-30.24) and in veterinary hospital patients (12.72 %; 95 % CI = 7.15-9.55). Differences in the prevalences between studies with indirect and direct diagnostic tests were noted in stray cats, owned cats and neutering campaign animals, while in veterinary hospital patients, the prevalences were similar (Table 5). In the variables recorded as factors associated with leptospirosis, both access to outdoors or street (14.90 %; 95 % CI = 10.45-19.99) and hunter / hunting rodent habit (11.38 %; 95 % CI = 4.24-21.24) have high pooled prevalences (Table 5).
Discussion
Zoonoses have impacts on human and animal health and although this is difficult to quantify, it can be assessed by epidemiologic studies with measures such as prevalence and incidence. There is a gap in the knowledge about their distribution, etiology, pathogens biology, hosts, dynamics, transmission cycle and risk factors.41 Leptospirosis is known to be one of the most relevant zoonosis worldwide, but at the same time, it has been a neglected disease since country surveillance systems do not always exist or are not effective.42 However, the re-emergence of Leptospira spp. in pet populations and the potential severity of this infection in humans and animals are reasons for concern.43
In this study, epidemiologic characteristics, and the global prevalence of leptospirosis in domestic cats were stated through a systematic review and meta-analyses. A total of 139 articles related with Leptospira or leptospirosis in domestic cats was found in the bibliographic search and 93 studies were selected for a full review according to their methodologic quality. Finally, 83 documents were included in M1 and 23 in M2 (Figure 1).
Regarding the geographic location, in studies performed with both diagnostic tests (direct and indirect), Asia showed higher pooled prevalences for leptospirosis (Table 2), highlighting that in those geographical areas, an increase in the awareness about the disease is needed, as well as to take prevention measures and to perform more epidemiological research, such as casecontrol or cohort studies. In general, epidemiologic studies are performed in all continents, which reflect an interest in the research about the disease in domestic cats.
The date of publication of the studies ranges from the 1940 decade until the year 2020. In studies with indirect diagnostic tests, the decades of 2000 and 2010 were the most frequent periods of publication, in contrast with a predominance of articles published in the decades 1970, 2010, and year 2020 on studies using direct methods (Table 3). This reveals that although there is epidemiological research on leptospirosis in cats, studies are concentrated on periods of time with an increasing trend since the decade of 2010. This is a period in which some clinical reports of leptospira infection in felines were published39, 44 describing lethargy, anorexia, weight loss, vomiting, diarrhea, respiratory signs, polyuria, polydipsia, and uveitis as clinical signs.5, 12, 45
According to some authors, the interpretation of MAT in cats can be more reliable than in dogs because no commercial vaccine against leptospira is available for felines, which reduces the likelihood of false positive results.35, 43, 46 Seropositivity to 19 serogroups and 36 serovars was found in this systematic review. The most frequent serogroups reported were Icterohaemorragiae (n = 9 studies), Australis, Autumnalis and Pomona (n = 7 articles) (Figure 2), and the serovars described mostly were Pomona (n = 27 articles), Grippotyphosa (n = 21 studies), Canicola (n = 20 documents) and Icterohaemorragiae (n = 19 studies) (Figure 3). Regarding this, domestic and wild mammal species can be maintenance or incidental host for leptospira,47 but it is not completely understood which serovars cause infections in cats, which requires more research.5
The identification of the most prevalent serogroups/serovars is essential to determine the sources of infection and the pathogenic Leptospira that can be related to the urinary shedding.13 Is important to consider that the selection of the serogroups/serovars to be evaluated by serology depends on the geographical location, therefore, a MAT panel should be constructed based on the knowledge of the frequency of presentation the serogroups locally45 and although the MAT have a good diagnostic specificity, the significance of a titer in a single sample depends on the frequency of the residual titers due to past infections48 Since crossreactions are common, serogroups should be used only to give a broad idea of the common serogroups present in a population.49 Moreover, a reaction with an individual serovar selected for use as an antigen representing a serogroup cannot be considered to suggest an infection with the serovar tested but, rather, infection with only an antigenically similar serovar of the same serogroup.50, 52
Only 11 articles described risk or protection factors associated with leptospirosis in cats (Table 4) out 139 document found in the bibliographic search, which can reflect that the analysis of factors associated with the disease is not performed with frequency, or is done, but the variables considered as risk or potential factors result are not statistically significant, as in the study by Peixoto et al.53 were variables such as age, sex, origin of the cats, breed, and presence of clinical signs were not associated with seropositivity for leptospirosis. In this systematic review and meta-analysis, the factors described as statistically significant in their respective studies, were related to environmental settings, lifestyle and maintenance conditions of the animals, age, and clinical chemistry parameters.
Azócar-Aedo et al.32 indicates that the probability leptospirosis transmission in cat populations can be influenced by factors such as management conditions of felines, habitat indoor, outdoor and interactions with feral cats or wild animals, which coincides with these results. The variables that are statistically associated with the disease or infection were different among studies. In fact, only two variables coincide: "access to outdoors or street" and "hunter/hunting rodent habit". An outdoor habitat is certainly a risk factor, considering that stray, feral or shelter cats are mentioned as more exposed to leptospires in one study.9 Moreover, the predation chain between cats and different rodent species could be linked to the seropositivity to serogroups Autumnalis and Ballum.54, 55
In the subgroup analysis, higher pooled prevalences were recorded in shelter, rural and outdoor cats in studies using indirect diagnostic tests, as well as in stray cats and in veterinary hospital patients in studies performed with direct diagnostic methods. Differences in the prevalences between studies with indirect and direct diagnostic tests were recorded in stray cats, owned cats, and neutering campaign animals. Otherwise, the access to outdoors or street and hunter/hunting rodent habit also showed elevated prevalences (Table 5). Since most of the knowledge of zoonoses related with pets relies on case reports, epidemiologic studies identify humanpet interactions in relation to the risk of disease are needed.43 Regarding leptospira, each country, region or city have epidemiological particularities related to maintenance and incidental hosts, climatic characteristics, and anthropogenic activities, which influence the risk of the disease.13 In humans, the infection, and outbreaks or even epidemics of leptospirosis are influenced by the interaction of environmental factors and the diversity of serogroups/serovars.56
Indirect diagnostic tests based on serology detect antibodies against Leptospira that can persist for long time, but do not necessary reflect present infections, while direct diagnostic tests identify the infectious agent itself. For this, two databases were constructed to perform M1 and M2.
It was demonstrated that seropositivity and infection is present in domestic felines, with prevalences between 9.22 (95 % CI = 4.30-15.41) and 11.09 % (95 % CI = 8.68-13.73). The lower prevalence estimated in studies with direct diagnostic tests was expected because urinary shedding to leptospira is manifested in a lesser extent than the antibody response to the bacteria according to Levett.57 A study in Japan reported a seroprevalence of antiLeptospira antibodies of 16.6 % of the cats tested and the leptospiral flaB gene was detected in 7.1 % of cat urine samples using PCR.58 In Vietnam, a seroprevalence of 12.2 % was reported,59 and leptospiral antibodies were detected in 10 % of feral cats from Canada,60 which coincide with our results.
The potential of leptospiruria does exist in cats and although there is no report of leptospirosis transmission from cats to humans, this possibility cannot be ruled out.5, 13 Sanhueza et al.61 stated that veterinarians spending from 50 % to 75 % of their time working with dogs or cats had more risk of being seropositive. Moreover, Barmettler et al.62 specified that seropositivity to leptospira among veterinary staff and pet owners exposed to dogs with acute leptospirosis can be infrequent if people follow standard hygiene protocols, however, updated epidemiologic research such as cross sectional and cohort studies are needed to increase scientific evidence regarding this. Considering the degree to which cat populations are increasing globally, it is important to take prevention measures in any disease with zoonotic potential transmission to humans.3
To achieve enhanced efforts in the control measures, coordination among animal and public health sectors are needed.63 Some measures to prevent the potential of leptospira infection in pet owners were described by Murillo et al.:5 1) to avoid contact with cat urine, 2) to wear protecting equipment and always wash one's hands after cleaning the cat litter box, 3) the use of chemical disinfectants to clean the cat litter box, as well as any other areas where the cat urinates, 4) the provision of prophylactic treatment to other pets in the same household that may have been exposed to leptospires in the environment.
Is important to note that leptospirosis represents a classic "One Health" problem that requires a deep knowledge of the transmission mechanisms, animal reservoir hosts involved, environmental sources of the organism, which can vary regionally and over time in different geographic areas worldwide.16 For this reason, it is important to continue conducting epidemiologic studies on leptospirosis in domestic cats, to increase and update existing information, considering that different prevalences are estimated depending on the geographic location of the study (continents) or the origins of the sampled cats, as was done in this systematic review and meta-analysis.
According to Brown and Sutton,25 and Easterbrook et al.,64 scientific journals are more likely to publish positive reports, in contrast to negative research findings, which lead high chances of publication bias in metaanalyses. The Begg and Egger tests can be used as statistical indication for this bias,65 which was detected in the M1 and M2, but the trim and fill method do not give robust results to estimate the number of studies needed to eliminate this, and this is a limitation of the present study. Other limitation was the heterogeneity noted in both M1 and M2, however, the geographic location of the study (continent), the origin of the sampled cats some and risk factors related with the disease were explored as the possibly sources of heterogeneity with a subgroup analysis (Table 5).
Conclusions
Observational epidemiologic research about leptospirosis in domestic cats is performed in all continents, as shown in studies published between the 1940 decade and the year 2020. Seropositivity in the studies is mainly described for Leptospira serogroups Icterohaemorragiae, Australis and Autumnalis and for serovars Pomona, Grippotyphosa and Canicola. The studies describe different risk or protection factors associated with the disease. The global prevalence of leptospirosis in cats ranges from 9.52 to 11.09 % in studies using direct and indirect diagnostic tests respectively, confirming seropositivity and infection and different prevalences depending on the geographic location of the study (continents) and the origins of the sampled cats. Since exposure and infection to leptospira is present worldwide, a potential disease transmission from cats to humans does exist, which is a public health concern and more epidemiologic research is needed, considering that domestic felines are common pets with close contact with people.
Data availability
All relevant data are within the manuscript.
Conflicts of interest
The author has no conflict of interest to declare in regard to this publication.
Author contributions
Conceptualization, data curation, formal analysis, methodology, writing-original draft, writing-review and editing: L Azócar-Aedo.











 nueva página del texto (beta)
nueva página del texto (beta)