Introduction
Analysis of the cellular component of blood is a common technique for evaluating the health status of wild animals.1,2 In tortoises, conditions such as anemia, leukemia, inflammation and allergy are associated with changes in blood cell values.3,4 While cell morphometry of numerous terrestrial, semiaquatic and marine tortoises has been previously described,5-7 there are still species of great ecological importance that are yet to be characterized.
Gopherus flavomarginatus is an endemic Mexican tortoise, considered to be the largest terrestrial chelonian in North America, with a carapace length of up to 40 cm.8 This species is listed as being in danger of extinction by the Official Mexican Standard 0599 and as critically endangered by the IUCN red list.10 The geographic distribution of this species is restricted to the Mapimí Bolson in the Mexican Chihuahuan desert, and has a protected status within the Mapimí Biosphere Reserve.11 Turtles of the genus Gopherus are key organisms for the ecosystems in which they live since they are herbivors performing crucial functions in seed dispersal.12 Moreover, the deep burrows that they excavate provide shelter for at least 300 invertebrate and 60 vertebrate species.13,14
The conservation efforts towards the G. flavomarginatus tortoise demand a better knowledge of its biology and health status. The present study aimed to determine the blood cell morphometry in this species, to provide information that may advance health monitoring, treatment and other protection strategies.
Methods
Study area
The Mapimí Biosphere Reserve covers an area of 342388 ha that includes part of the states of Chihuahua, Coahuila and Durango in Mexico (26°00' and 26°10'N and 104°10' and 103°20'W). The climate is very arid, semi-warm BWhw(e), with an average annual temperature of 25.5 °C and summer rains.15 The predominant vegetation is rosette and microphile scrubs, as well as halophyte, and gypsophila plants.16
Field work
From May 2015 to September 2017, forty-four individuals of the G. flavomarginatus species were captured within the Mapimí Biosphere Reserve. Blood samples were drawn from the subcarapacial vein following the USFWS protocol (Figure 1).17 Prior to sampling, the puncture area was cleaned with a cotton swab dipped in alcohol. A 3 mL syringe with a 23 x 25 mm gauge needle was then used to extract one milliliter of blood. Samples were subsequently placed in 6 mL BD Vacutainer® tubes with lithium heparin and stored in a cooler at approximately 4 °C until processed. Gender was recorded for each captured tortoise considering a flat plastron, short tail, and absence of glands under the chin for females, and a concave plastron, longer tail and presence of glands under the chin for males.18 Health of all individuals was determined by established protocols 17,19 before their release at the site of capture.
Tortoise samples were collected under the DGVS 07249/15-16-17 permit granted by SEMARNAT, Mexico.
Cell morphometry
Blood samples were processed within the first three hours of collection. Two
smears from each sample were prepared on glass slides and stained with Wright's
dye (Analytyka®). Blood cell types of G. flavomarginatus were
characterized following criteria previously set for reptiles.5,6,20-22 The center of the smear was
chosen for observation of cell characteristics since it was where cells appeared
in well-defined monolayers.23
Shape and color of cells were recorded by means of an Axiocam ERc5s Carl Zeiss®
microscope and camera. One hundred erythro-cytes, 30 heterophils, 30
eosinophils, 30 basophils, 30 lymphocytes, 30 mono-cytes and 30 thrombocytes
were measured for each gender. Cell length (CL) and width (CW) were recorded for
erythrocytes, heterophils, eosinophils, monocytes and thrombocytes, as well as
the length and width of their nuclei (NL and NW, respectively). For basophils
and lymphocytes, only length and width of cells were documented. All
measurements were determined with the ImageTool 3.0 software, and total cell
size (μm2) was calculated using the formula
Statistical analyses
Normality and homoscedasticity of data were assessed by the Shapiro-Wilk and Levene tests, respectively (P < 0.05).The x' = log (x + 1) transformation was used when needed.25 Student t tests were used to determine independent variable differences between gender (P < 0.05). Statistical tests were performed with the PAST 3.14 software.26
Results and discussion
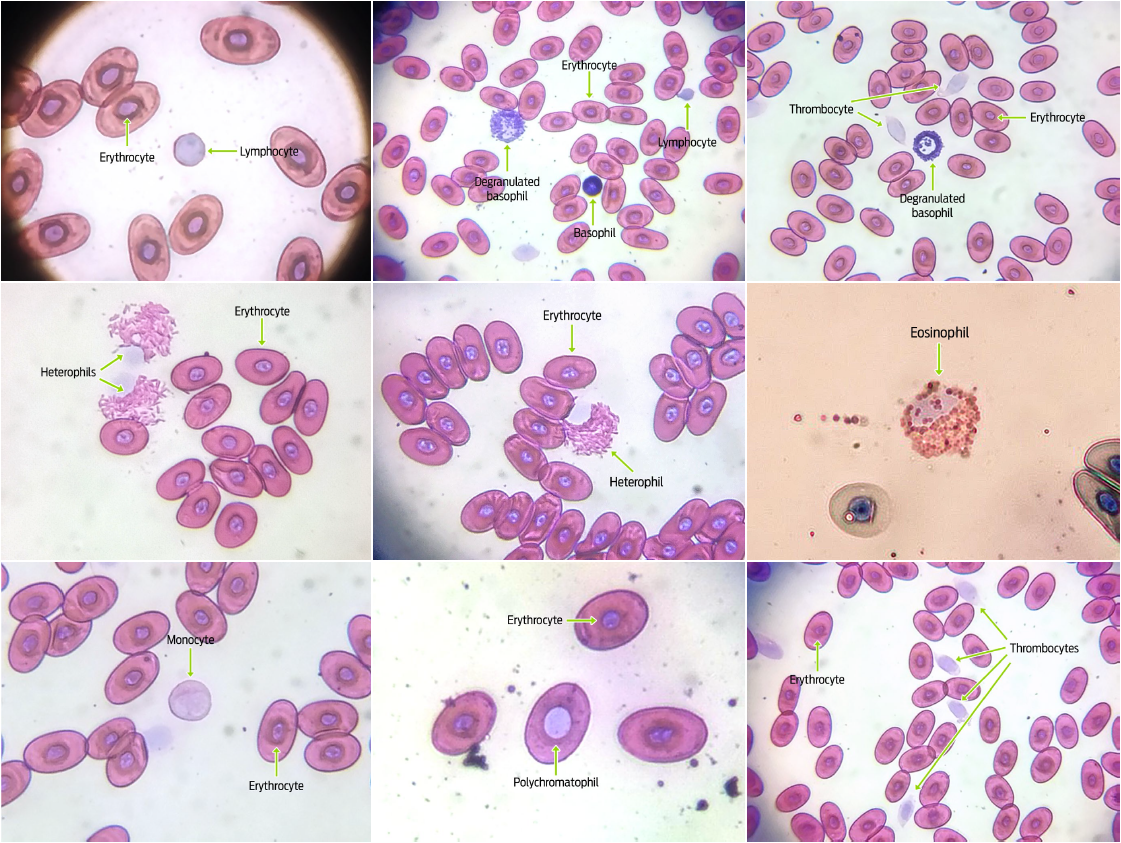
Blood samples were obtained from forty-four G. flavomarginatus individuals (16 males, 28 females). Sizes found for all blood cells fell within previously documented values for reptiles.6,30 The shape and color of erythrocytes, leukocytes and thrombocytes of G. flavomarginatus were similar to those described for G. agassizii, and G. polyphemus tortoises.20,27-29 Erythrocytes showed an elliptical shape and smooth edges, with a central oval-shaped nucleus (Figure 2). The cytoplasm, which occupied a large surface area when compared to the nucleus, showed an acidophilic coloration (pink color), whilst the nucleus was dyed deep purple. Size of erythrocytes was similar between females (216.06 ± 24.0 μm2) and males (215.46 ± 24.2 μm2) (P = 0.862; Table 1). Nuclear size of erythrocytes was also analogous for both sexes (19.96 ± 5.1 μm2, and 18.95 ± 4.8 μm2, for females and males, respectively) (P = 0.156; Table 1). Most of the erythrocytes observed in the slides were mature and presented with the previously described morphology, however, larger round polychromatophilic cells were also apparent (Figure 2). Alleman et al. described polychromatophilic erythrocytes in G. agassizii tortoises.20 Indeed, this type of cell, albeit circulating in low quantities (< 1%), appears to be commonly found in healthy reptiles, particularly in juvenile or molting individuals. 6
Table 1 Length and width of blood cells (μm2) in male and female Gopherus flavomarginatus tortoises.
| Gender | Measurement | Erythrocyte | Heterophil | Eosinophil | Basophil | Lymphocyte | Monocyte | Thrombocyte |
| Male | CL | 21.6 ± 1.0 | 17.7 ± 2.0 | 17.6 ± 1.4 | 11.7 ± 1.1 | 7.4 ± 0.8 | 16.7 ± 2.0 | 10.5 ± 1.0 |
| CW | 12.3 ± 0.9 | 15.6 ± 2.2 | 15.6 ± 2.2 | 11.6 ± 1.1 | 7.3 ± 0.8 | 15.4 ± 2.4 | 9.6 ± 1.2 | |
| NL | 6.0 ± 0.8 | 7.8 ± 1.1 | 8.3 ± 1.4 | — | — | 10.2 ± 2.2 | 6.9 ± 1.1 | |
| NW | 4.5 ± 0.6 | 6.3 ± 0.9 | 6.2 ± 0.9 | — | — | 7.6 ± 1.7 | 5.1 ± 0.8 | |
| Female | CL | 22.4 ± 1.5 | 17.8 ± 1.8 | 17.6 ± 1.0 | 11.1 ± 1.1 | 7.8 ± 1.3 | 17.0 ± 1.9 | 10.6 ± 1.1 |
| CW | 12.5 ± 1.0 | 15.8 ± 2.0 | 15.6 ± 1.7 | 11.1 ± 1.4 | 7.7 ± 1.2 | 15.3 ± 2.2 | 9.5 ± 1.1 | |
| NL | 5.7 ± 0.6 | 7.7 ± 1.0 | 8.8 ± 1.3 | — | — | 10.3 ± 2.2 | 7.0 ± 1.0 | |
| NW | 3.8 ± 0.5 | 6.3 ± 0.9 | 6.4 ± 0.9 | — | — | 7.7 ± 1.7 | 4.9 ± 0.7 |
CL: cell length; CW: cell width; NL: nucleus length; NW: nucleus width. Data are presented as mean ± standard deviation.
Granular leukocytes: heterophils, eosinophils, and basophils were also observed in smears. Heterophils showed a spherical shape with a large quantity of round and rod-like cytoplasmic granules that stained orange-pink. The lilac colored nucleus was round or oval, and eccentrically located (Figure 2). Sizes of these cells and their nuclei were similar for both males and females (heterophil cell size: 223.79 ± 47.5 μm2 in females, and 219.82 ± 49.5 μm2 in males, P = 0.753, heterophil nuclear size: 223.79 ± 47.5 μm2 in females, and 219.82 ± 49.5 μm2 in males, P = 0.864; Table 1). While the morphology and coloration of eosinophils were similar to that seen for heterophils, the cytoplasmic granules in the former cells were completely spherical (Figure 2). Eosinophil cell and nuclei sizes were comparable between sexes (cell size: 216.80 ± 26.7 μm2 in females and 217.51 ± 38.6 μm2 in males, P = 0.934; nuclear size: 45.24 ± 10.1 μm2 in females and 41.24 ± 10.5 μm2 in males, P = 0.138; Table 1). Round-shaped basophils had a large number of intensely basophilic (purple) cytoplasmic granules that mostly covered the nucleus completely. When the nucleus was visible, it was round and either central or eccentric (Figure 2). Degranulated basophils could also be detected in blood smears. No differences in basophil morphometry were found between sexes (cell size: 99.66 ± 26.1 μm2 in females and 108.56 ± 20.4 μm2 in males, P = 0.147; Table 1). The morphological similarity between heterophils and eosinophils is well-known for most reptile species, and it is the differences of their cytoplasmic granule shapes that allows for the differentiation between both cell types.30 Accordingly, heterophil granules were rod-like shaped, while eosinophil granules were round in G. flavomarginatus smears.
The detected non-granular leukocytes were lymphocytes and monocytes. Lymphocytes were characterized by being round-shaped, with a pink stained cytoplasm, and a large pale purple nucleus that occupied most of the cell (Figure 2). No gender differences were observed for lymphocyte size (49.04 ± 16.2 μm2 in females, and 42.88 ± 9.2 μm2 in males, (P = 0.075; Table 1). Monocytes had a round or amoeboid shapes and a pleomorphic nucleus (which could be round, oval, kidney-shaped or lobed). Both the cytoplasm and the nucleus stained pale pink, with the latter being paler than the former (Figure 2). No differences were found for monocyte cell or nuclear sizes between sexes (cell size: 207.66 ± 43.0 μm2 in females, and 204.47 ± 43.3 μm2 in males, P = 0.776; Nuclear size: 62.0 ± 15.5 μm2 in females, 60.87 ± 15.6 μm2 in males, P = 0.780, Table 1). Monocytes were the scarcest type of leukocytes in G. flavomarginatus blood smear samples, and were characterized as type 1 monocytes. Conversely, there are two types of monocytes that can be found in Gopherus agassizii tortoises: type 1 is the most abundant and its morphology is very similar to that found in mammals, with a single irregularly shaped nucleus, moderate amounts of cytoplasm (of a pale pink tone) and one or more light colored vacuoles; type 2 monocytes are similar to type 1 but the nucleus is lobed or pleomorphic, with small amounts of small cytoplasmic granules of a reddish purple coloration, which is why they are called azurophil monocytes.20 These latter cells are rare in chelonians, but common in snakes, lizards and crocodiles, where they contribute to inflammatory reactions.6,28,31
Finally, thrombocytes were found to have an oval or round shape. The nucleus was ellipsoid and stained either pink or light purple. The cytoplasm was observed surrounding the nucleus, with bright hyaline material (Figure 2). Thrombocyte morphology was similar for males and females of the G. flavomarginatus species (cell size: 79.96 ± 13.7 μm2 in females, and 79.97 ± 15.7 μm2 in males, P = 0.998; nuclear size: 27.38 ± 5.2 μm2 in females and 28.18 ± 6.6 μm2 in males, P = 0.602; Table 1). Most thrombocytes were easily recognized because they tended to agglutinate, however, when observed individually, a transparent, even shiny, cytoplasm with spike-like extensions could be seen. Similar characteristics have been previously described for G. agassizii thrombocytes.20
Conclusions
This work describes the blood cell morphology of wild Bolson tortoises in the Mexican Chihuahuan desert. The data obtained from this study advances the knowledge of the biological traits of G. flavomarginatus, and is thus instrumental towards efforts to monitor the health status of captured individuals, as well as for the development of conservation strategies aimed to protect this endangered species.











 nueva página del texto (beta)
nueva página del texto (beta)