Introduction
Anesthesia has critical importance in avian medicine and surgery. In poultry, medetomidine, xylazine, midazolam, diazepam, butarphanol, atropine and glycoprolate are commonly used as pre-anesthetics drugs.1 Medetomidine is a potent and selective α-2 adrenoreceptor agonist, but it is also one of the safest premedication agent used in birds.2 When applied by intramuscular injection (25 μg/kg), it causes sedation and loss of reflexes in 8-day-old chicks.3 Ketamine, an injectable cyclohexane, is a dissociative drug that produces poor muscle relaxation in animals, but that has been deemed suitable as an anesthetic agent for birds.4,5 However, it can only be used on its own for short procedures, since it can cause muscle tremors, catalepsy (muscle stiffness), myotonic contractions, opisthotonus and rough recovery periods.5,6 Midazolam has a relatively rapid metabolic clearance compared to the other benzodiazepines and hence has a short duration of action in all species. It has anticonvulsant, anxiolytic, sleep-inducing, muscle relaxing, and sedating effects when administered orally, intramuscularly, or intravenously.7,8 When combined with ketamine, a quick onset and deep anesthesia, with good muscle relaxation and a reduced recovery time was attained in broiler chicks.9 Isoflurane and sevoflurane are commonly used inhaled agents that are also used in birds for induction maintenance of anesthesia.10 Sevoflurane has a lower blood/gas solubility coefficient than isoflurane. It also has shorter recovery times compared to other anesthetic drugs in long-term anesthesia.11 It does not affect heart rate in chickens, and results in less apnea and irritation when administered with a mask.12
Surgical procedures are frequently performed in poultry, and an adequate combination of anesthetics is valuable for clinical practice. In this study, we examined the effects of a combination of medetomidine, midazolam, ketamine and sevoflurane anesthesia on physiological and biochemical parameters in chickens.
Materials and methods
Animals
Twenty, 72-day old healthy Lohmann Brown chicks, with a body weight of 1.7 ± 0.3 kg were used for this study. To reduce handling stress, all daily management procedures, as well as sampling and testing protocols (blood collection, injections, and electrocardiography) were performed by the same person.
This study was approved by the Erciyes University local Ethics Committee and complied with the International Guidelines for the use of animals in Biomedical Research.
Anesthesia preparation
There were no feeding restrictions for animals before anesthesia. The dose of the anesthetic agent was calculated for each individual based on its weight. Chicks were left alone to provide for a relaxing environment for 10 minutes before applying the pre-anesthetic agent. Baseline values were taken immediately before anesthesia (time point 0 / T0) and recorded in an examination form.
Anesthesia protocol
A combination of midazolam (MID) (0.5 mg/kg, Zolamide 5 ml/5 mg 5 amp, Defarma, Turkey) and medetomidine (MED) (50 μg/kg, Domitor 10 ml, Pfizer, Finland) was injected into the pectoral muscles of each chicken mixed with 1 ml of saline solution (0.9% NaCl) as preanesthetic [time point 1(T1)]. Ten minutes later a 25 mg/kg (Ketasol 10%, 10 ml, Interhas, Turkey) of ketamine-HCl (KET) was IP applied [time point 2 (T2)]. After a further 10 minutes, chicks were intubated with silicone tubes (2-3 mm in diameter) to maintain anesthesia for 30 min using 2% sevofluorane (SEVO) (Sevorane Liquid 100%, 250 cc, Abbvie, England) with 500 ml/min oxygen flow [time point 3 at end of these 30 minutes (T3)]. An SMS 2000 Classic inhalation anesthesia device applying the modified Jackson-Rees (Ayres T piece) system was used.
Electrocardiographic examination
Electrocardiographic findings were recorded (CAREWELL 1103G Veterinary ECG Device) at T0 before anesthesia and at time points T1, T2 and T3 during anesthesia. Bipolar extremity (I, II, III) and increased unipolar (aVR, aVL, aVF) limb leads were used for electrocardiograms with the aid of crocodile electrodes. ECG parameters and intervals were recorded at derivation II, at a 25 mm/s speed.
Clinical examination and reflex response assessment
Changes in palpebral and pedal reflexes, as well as head, neck, wing and body position alterations were recorded for all anesthesia time points. Also, cloacal body temperature (°C) and heart-respiratory rates were concurrently recorded.
Reflex responses were graded according to the following criteria, as has been previously reported:13
Biochemical analyses
Blood was drawn (3 ml) from the vena brachialis of each individual into tubes without anticoagulant at T0 (baseline) and at T3 for biochemical analyses. Blood was centrifuged at 1500 rpm for 15 minutes to obtain sera. Concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), creatinine (CR), and glucose (GLU), as well as total protein and LDH were assessed.
Statistical analyses
The data was evaluated with the statistical package program IBM SPSS Statistics 22.0 (IBM Corp., Armonk, New York, USA). Values are reported as mean ± standard deviation (SD) and median (min-max) values. Normal distributions of data were evaluated by the Shapiro Wilk normality test and Q-Q graphs. Paired t and Wilcoxon test were used when two consecutive measurements were assessed. Analysis of variance for repeated measures and the Friedman test were used when four successive values were assessed. In case of a difference, parametric and nonparametric Student-Newman-Keuls multiple comparison test was used. Statistical differences were set at p < 0.05.
Results
Physiological parameters
Mean cloacal body temperature decreased and remained low throughout anesthesia (T1, T2, T3 and recovery vs T0; p < 0.05). Respiratory rate was lower at T1, T2 and T3, when compared to T0 (p < 0.05) but partially rose during recovery time. Similarly, heart rate decreased gradually during anesthesia (p < 0.05) but increased during reanimation. Mean values of physiological parameters are shown in Table 1.
Table 1 Physiological parameters before, during and after anesthesia (mean ± SD)
| Parameters | T0 | T1 | T2 | T3 | Reanimation |
| Cloacal temperature (°C) | 41.27 ± 0.28a | 40.91 ± 0.51b | 40.33 ± 0.45b | 39.17 ± 0.80c | 40.13 ± 0.65b |
| Heart rate | 242.70 ± 37,60a | 204.90 ± 27.16 b | 203.10 ± 37.83b | 194.60 ± 39.68c | 208.73 ± 36.40b |
| Respiratory rate | 43.80 ± 7.73a | 22.80 ± 6.87b | 21.40 ± 9,64b | 16.10 ± 5.74c | 24 ± 6.44b |
a,b,c Different letters shows statistically significantly difference in the same line (p < 0.05).
SD: standard deviation; T0: before anesthesia; T1: 10 minutes after injection of medetomidine-midazolam; T2: 10 minutes after injection of ketamine; T3: 30 minutes after administration of sevoflurane.
Electrocardiographic examination
Tachycardia was observed in all chickens during the preanesthetic period. Sinus tachycardia and ventricular tachycardia were the most common findings observed during general anesthesia in ECG records. Mean amplitude of the P wave increased at T1 and T2 (p < 0.05), but partially decreased by T3 (Table 2). As for mean PR time values, there was an increase at T1 that returned to the baseline values by T2 and remained unchanged thereafter. There were no differences for the QRS time interval. The mean ST interval increased at T1 and remained higher than baseline values at T2 and T3 (p < 0.05). The QT and T wave amplitude values did not differ from T0 at any time during anesthesia. Mean values for ECG parameters are presented in Table 2. Examples of ECG prints during anesthesia are shown in Figure 1.
Table 2 ECG parameters before, during and after anesthesia mean ± SD
| ECG Parameters | T0 | T1 | T2 | T3 |
| P amp (mv) | 0.02 ± 0.11a | 0.15 ± 0.04b | 0.78 ± 0,28b | 0.4 ± 0.05c |
| P time (s) | 0.03 ± 0.18 | 0.04 ± 0.07 | 0.02 ± 0.0 | 0.02 ± 0.00 |
| PR time (s) | 0.06 ± 0.07 | 0.09 ± 0.16 | 0.07 ± 0.08 | 0.06 ± 0.01 |
| QRS time (s) | 0.06 ± 0.09 | 0.03 ± 0.00 | 0.03 ± 0.0 | 0.04 ± 0.01 |
| ST time (s) | 0.04 ± 0.03a | 0.08 ± 0.01b | 0.09 ± 0.01b | 0.13 ± 0.17b |
| QT time (s) | 0.11 ± 0.08 | 0.12 ± 0.01 | 0.12 ± 0.02 | 0.13 ± 0.01 |
| T amp (mv) | 0.19 ± 0.16 | 0.25 ± 0.12 | 0.21 ± 0.09 | 0.22 ± 0.09 |
a,b,cDifferent superscripts within the same row indicate statistical differences (p < 0.05).
amp: amplitude; ECG: electrocardiogram; SD: standard deviation; T0: before anesthesia; T1: 10 minutes after injection of medetomidine-midazolam; T2: 10 minutes after injection of ketamine; T3: 30 minutes after administration of sevoflurane.
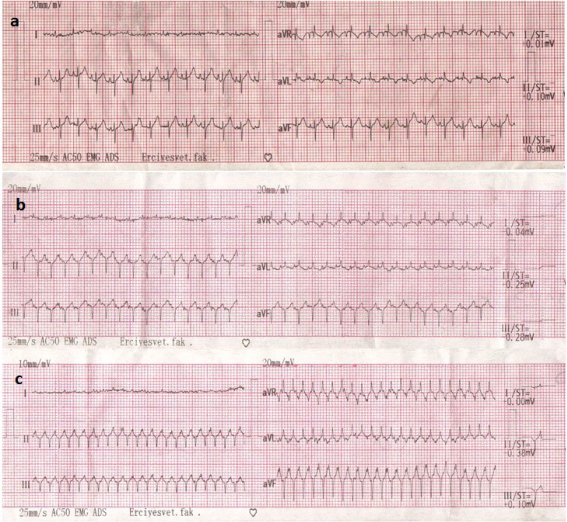
Figure 1 Examples of electrocardiogram prints showing different parameter results from anesthetized chicks. a) Normal sinus rhythm after medetomidine and midazolam administration, ST segment elevations. b) Sinus tachycardia after ketamine administration. c) Ventricular tachycardia after sevoflurane administration.
Clinical findings
Reflexes were lessened from anesthesia onset (T1) and later lost, to be recovered by the reanimation period in all individuals (p < 0.05). The palpebral reflex was still present in 4 of the 20 chicks at T1 but had already lessened in the rest of the animals by this time point. By T2, the palpebral reflex had completely disappeared in 15 individuals. At T3 there was no sign of this reflex in any of the anesthetized animals. The pedal reflex at T1 remained strong in 3 chicks, lessened in 14 animals and was completely lost in the other 3 individuals. By T2, no signs of this reflex were observed in 18 chicks. Wing reflex was present in one, lessened in 11, and absent in 8 chickens at T1. By T2, nineteen animals had a decreased wing reflex, that was absent in all animals by T3. Grading of reflex responses is presented in Table 3.
Table 3 Reflex response grading at different time points during the anesthetic protocol (median)
| Clinical reflex | T0 | T1 | T2 | T3 | Recovery |
| Wing withdrawal | 1a | 2a | 3a | 3a | 1 |
| Palpebral reflex | 1a | 2a | 3a | 3a | 1 |
| Pedal reflex | 1a | 2a | 3a | 3a | 1 |
aIndicates differences from T0 within the same row (p < 0.05).
T0: before anesthesia; T1: 10 minutes after injection of medetomidine-midazolam; T2: 10 minutes after injection of ketamine; T3: 30 minutes after administration of sevoflurane.
The presence of the palpebral reflex, as well as eye opening, were considered to determine recovery time, which lasted between 45 and 270 min (mean= 125.75 min). No differences were found for the pedal and palpebral reflexes, or withdrawal of wings when T0 and recovery time were compared.
Biochemical findings
Mean values for ALT, CRE, LDH, ALB, GLU, total protein (TP), and AST at T0 and T3 are presented in Table 4. No differences were found between these time points for TP, AST, CRE or LDH. Conversely, albumin, ALT, and GLU were lower at T3, when compared to T0 (p < 0.05).
Table 4 Biochemical parameters of anesthetized chicks at T0 and T3 mean ± SD
| Biochemical parameters | T0 | T3 |
| Albumin (g/dl) | 2.05 ± 0.41a | 1.72 ± 0.49a |
| AST (IU) | 4.667 ± 1.617 | 3.600 ± 1.590 |
| Glucose (mg/dl) | 294.65 ± 69.97a | 257.10 ± 46.97a |
| Total protein (g/dl) | 5.97 ± 1.19 | 5.71 ± 1.55 |
| ALT (IU) | 0.028 ± 0.017a | 0.006 ± 0.010* |
| Creatinin (mg/dl) | 0.22 ± 0.07 | 0.20 ± 0.0 |
| LDH (mg/dl) | 467.95 ± 349.21 | 544.95 ± 358.01 |
a Differences between T0 and T3 within the same row (p < 0.05).
ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; SD: standard deviation; T0: before anesthesia; T3: 30 minutes after administration of sevoflurane.
Discussion
The anesthetic protocol used for chickens in this study was safe, with a rapid induction period and a smooth recovery interval. Respiratory rate decreased following premedication and remained lower than baseline values (T0) through later stages of anesthesia and the recovery period. However, there were no irreversible complications. This decrease is believed to have derived from an increased level of circulating catecholamines after anesthetic administration. Moreover, a negative effect of medetomidine on the respiratory system has been previously reported in ducks.14,15
The mean heart rate of animals during anesthesia in this study was lower than that reported as normal for chicks of over 20 weeks of age (249 ± 7.0 beats per minute).16 There was a sharp descent in mean heart rate after the injection of preanesthetic agents (T1) that continued throughout the anesthesia period. By reanimation, this value was partially recovered, but remained lower than the normal heart rate in chickens of 250-300 beats per minute.17,18No changes in mean heart rate were found during anesthesia in chickens that were subjected only to a sevoflurane protocol.19 However, the heart rate of pigeons, decreased suddenly during pre-anesthesia and continued to descend gradually when anesthetized with medetomidine and ketamine.2 Similarly, heart rate was reduced in domestic pigeons and White Leghorn cockerels after medetomidine-ketamine or xylazine-ketamine administration.20,21There have been reports of transient hypertension, bradycardia, and apnea, as well as the need of resuscitation maneuvers when medetomidine, midazolam, ketamine and propofol combinations were used in ducks.15 However, no critical situations arose with the anesthetic agent combination used in this study.
General ventricular tachycardia was observed in chicks before anesthesia, probably as a consequence of stress due to handling.23 Ventricular tachycardia was also observed in some individuals while under anesthesia, probably due to myocardial hypoxia.24
When anesthetized, avian species T waves become smaller in the ECG and eventually disappear, whilst R waves increase and S waves decrease in magnitude.24 The most significant changes observed in the ECG in this study were an increase in both the P wave amplitude and the ST time. Bahri et al. observed an inverse correlation between heart rate and P wave interval in chickens.17 Expansion of P waves has also been observed in atrial depolarization abnormalities such as hypertrophic myopathies, systemic hypertension, and mitral stenosis.25,26The increase in P wave amplitude found for animals in this work may be associated with dyspnea induced by the anesthetic agents. As for the ST segment, it may often be absent or short in healthy pigeons and parrots. In this study, high ST time values could be an effect of myocardial hypoxia.
One of the most common problems during poultry anesthesia procedures is hypothermia, which increases the recovery period.27 The decrease in cloacal body temperature are thought to be related to the vasodilating effects of the anesthetic agents as well as the physiological characteristics of the animals (hypotension, bradycardia and tachypnea).22 Nonetheless, the decrease in cloacal temperature that occurred following preanesthetic agent injection in animals in this study, recovered to approach baseline values (39.17 ± 0.80 °C) by the end of sevoflurane administration (T3).
Similar to results from our work, pigeons exposed to medetomidine, butorphanol, or ketamine have been reported to have good muscle relaxation and adequate analgesia.13,28In the present study, eyes were closed following premedication and the palpebral reflex disappeared after KET and SEVO administration in all chickens. Previous studies with caged birds report that the palpebral reflex remains present when the level of anesthesia is mild, and then disappears at moderate or deeper levels.29 Recovery periods have been found to span between 126 and 135 minutes in chicks undergoing sevoflurane anaesthesia.30 Animals from this study presented longer recovery times (mean 125.75 min, range 45-270 min).
Glucose levels can increase after sevoflurane anesthesia in humans and goats, or isoflurane anesthesia in eagles.31,32It has been suggested that this may be related to an inhibition of insulin secretion, or to a high metabolic rate in birds.32,33Levels of glucose were lower at T3 than at T0 in animals in this study, which could be attributed to a lower metabolic rate in chickens when compared to wild birds.
Most anesthetic agents are metabolized in the liver and assessment of the ALT enzyme, as well as other biochemical parameters can be used as an indication of hepatic health.4,31In the present study, neither creatinine nor LDH levels were altered but ALT concentrations were lower at T3 when compared to baseline values. These findings were considered to be due to the effects of catecholamines, anesthetic stress, and hepatic metabolism of anesthetic agents.
Conclusion
Mild changes in glucose levels (hyperglycemia), body temperature, and heart and respiratory rates (tachycardia and dyspnea) were observed after a MED + MID + KET + SEVO anesthesia protocol in chickens. However, none of these alterations were critical or life-threatening. Therefore, the MED + MID + KET + SEVO combination can be used to obtain satisfactory anesthesia in chickens. Further studies are needed to fully assess the use and qualities of this drug combination for anesthesia protocols in this and other bird species.











 text new page (beta)
text new page (beta)



