Introduction
The feline panleukopenia virus (FPV) and the canine parvovirus (CPV) are single-stranded DNA members of the Parvovirinae subfamily of Parvoviridae.1 They are antigenically related to each other and to the mink enteritis virus (MEV), which similarly to the CPV, has an approximately 5,000 base pair (bp) genome. Canine parvoviruses (variants CPV-2a, -2b and -2c) have been isolated from healthy and feline-panleukopenia-like symptom-exhibiting cats.2,3 Although CPV-2 differs from FPV in only a few amino acids, this discrepancy suffices to alter its pathogenesis, as disease induced by this virus evolves much more rapidly than FPV in cats.4 Although DNA viruses have a very low tolerance for mutation, CPV shows a nucleotide substitution rate closer to that seen for RNA viruses.5 The genome of parvoviruses contains two open reading frames (ORF), of which ORF1 encodes the non-structural proteins NS1 and NS2; and the smaller ORF2 encodes the structural proteins VP1 and VP2.6 The VP2 protein is a major capsid component that is significant for both viral pathogenicity and host immune reaction.7 The CPV-2a and -2b variants differ from the original CPV-2 strain in five or six amino acid residues of the VP2 capsid protein.2
The FPV was first recognized in the 1920s, whereas CPV was identified as an infectious agent in canines until the late 1970s. It is thought that CPV-2 originated from FPV (which only infects cats), with the CPV-2a, -2b and -2c antigenic variants appearing subsequently.8 While CPV-2 replicates in dog and cat cells, FPV reproduces efficiently only in feline cells.9 Several species of cats, minks, raccoons and foxes can be infected with FPV.12 Parvoviruses that cause acute gastroenteritis are very contagious and are often fatal, the severity of symptoms varying with age and immune system status of the host.10-12
Due to their contagious nature, parvoviruses are widespread throughout the Turkish cat population.13-15 Both FPV and CPV-2 (and its variants: 2a, -2b and -2c) occur in Turkey, with FPV having the highest prevalence (45.5%).13-15 Further, an FPV-specific antibody was detected in 16% of 151 Turkish cats between three months and six years of age.16 Nonetheless, studies on CPV-induced diseases in felines are limited in Turkey, and there is a need to update existing information on parvovirus molecular characterization and prevalence. Thus, the current study aimed to characterize the phylogeny of parvoviruses isolated from Turkish cats with clinical symptoms. To our knowledge, this is the first study to report nearly full- length VP2 gene sequences from parvovirus strains obtained from symptomatic domestic Turkish cats.
Materials and methods
Biological samples
Blood was collected from four cats in vacuum tubes containing EDTA. Also, an abdominal exudate was obtained from a fifth animal. Biological material was subsequently sent to the laboratory for viral diagnosis. Sampled cats presented clinical symptoms that included gastroenteritis, diarrhoea, fever, vomiting, and loss of appetite. All animals resided in Ankara, Turkey, and were either stray (FPV/TR/2017/ cat1), household (FPV/TR/2017/cat2 and FPV/TR/2017/cat5) or owned outdoor cats (FPV/TR/2017/cat3 and FPV/TR/2017/cat4). Both owned outdoor animals lived together. The abdominal exudate was taken from the FPV/TR/2017/cat5, that had coronavirus-like symptoms. Both FPV/TR/2017/cat4 and FPV/TR/2017/cat5 were previously vaccinated against FPV.
DNA extraction and VP2 gene amplification
Biological samples were used for total DNA extraction according to the phenol:chlo- roform:isoamyl alcohol method, as described by Sambrook and Russell.17 The DNA pellets were resuspended with 20 µl of deionised water and used as a template for PCR. Pellets were stored at -20 °C until testing. For partial VP2 gene amplification, sense and antisense oligonucleotide primers that targeted a 427-bp were used (5′-CTTTAACCTTCCTGTAACAG-3′, nucleotides 4043-4062 and 5′-CATAGT- TAAATTGGTTATCTAC-3′, nucleotides 4449-4470, respectively), as described by Pereria et al.18 The PCR was performed in a 30 µl reaction containing 5 U/µl Taq DNA polymerase (MBI, Fermentas, Waltham, MA, USA), 10 × Taq Buffer [including (NH4)2SO4, 25 mmol/L of MgCl2, and water 18 Mohm/cm, Applichem, Darmstadt, Germany], the primers, and 3 µl of DNA extracted from samples. An initial denaturation step at 94 °C for 10 min was followed by 40 cycles of DNA denaturation at 94°C for 30 s, primer annealing at 50 °C for 30 s and amplification at 72 °C for 60 s. Subsequently, reactions were incubated at 72 °C for 10 min for final extensions. For nearly full-length VP2 gene amplification, P1 and VP reverse primers were designed for both feline and canine parvovirus (5’-ATGAGTGATG- GAGCAGTTC-3’ and 5’-TTCTAGGTGCTAGTTGAG-3’, respectively).19 These primers produced a 1745-bp product from the parvovirus VP2 protein. PCR fragments were generated using Phusion High-Fidelity DNA Polymerase (Invitrogen, Germany) in a 20 µl reaction. The cycling protocol was 35 cycles of denaturation at 98 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 1 min, followed by a final extension at 72°C for 10 min.
Cloning and phylogenetic analyses
Amplicons obtained from partial VP2 PCR for each sample were purified using the Gene JET PCR Kit (Thermo Scientific, Waltham, MA, USA). Clean products were blunted and ligated to the pJET1.2 vector provided by the CloneJet PCR Cloning Kit (Thermo Scientific, Foster City, CA, USA), and subsequently used to transform cells, according to the manufacturer’s instructions. The plasmid containing viral DNA was then isolated using the PureLink® Quick Plasmid Miniprep Kit (Invitrogen) and sent to BM Labosis (Ankara, Turkey) for Sanger sequencing. Amplicons were characterised by bidirectional sequence reading to reduce errors. For the phylogenetic analysis of the VP2 gene, 72 partial and 38 full-length feline and canine parvovirus sequences from different regions of the world were obtained from GenBank. All sequences were aligned using The CLC Main Workbench v5.5 tool (CLCBio, Aarhus, Denmark), and analyzed with the MEGA 6.06 software.20 Maximum-likelihood (ML) trees were generated by the Kimura two-parameter model, with 1,000 boot- strap replicates (Figure 1).

Figure 1 A) Molecular phylogenetic analysis of the partial VP2 gene sequence (427 bp) by the maximum likelihood method. Sequences obtained in this study localized within CPV-like parvovirus strains. B) Molecular phylogenetic analysis of the nearly full-length VP2 gene sequence (1530 bp) by the maximum likelihood method. Sequences obtained in this study constituted a cluster in FPV-like parvoviruses and drew a separate monophyletical branch. The evolutionary history was inferred based on the Kimura 2-parameter model for both trees.
Nearly full-length VP2 sequences from this work were submitted to the NCBI GenBank database. Nucleotide sequences were deposited under accession numbers MH496766, MH496767, MH496768, MH496769 and MH496770 for FPV/R/2017/cat1, FPV/TR/2017/cat2, FPV/TR/2017/cat3, FPV/TR/2017/cat4 and FPV/TR/2017/cat5, respectively. The rates of nucleotide identities and similarities were calculated with the MatGAT 2.01 application.21 A linear regression of root-to- tip genetic distance was also developed, as implemented in TempEst, to infer the potential evolutionary rate across the phylogeographic tree.22
Results and discussion
The samples screened by PCR yielded a specific 427-bp amplicon for the partial VP2 sequence and a 1,530-bp product for the near full-length gene sequence. The abdominal exudate from the FPV/TR/2017/cat5 was found to be positive for FPV and negative for feline coronavirus. BLAST searches revealed that the sequences obtained from the blood and exudate samples were 95-99% identical to sever- al parvovirus strains. Two kinds of phylogenetic trees were generated based on the VP2 nucleotide sequence alignments. One of these trees contains 72 partial genomes, and the other 38 full-length genomes. Phylogeographic analyses of all strains with nearly full-length VP2 sequences, revealed that four of the sequences obtained from the sampled cats were grouped in the FPV cluster, whilst the fifth sequence (FPV/TR/2017/cat3), also part of this clade, was placed in a distinct branch (having a 99.2-99.3% nucleotide similarity with the other four field strains) (Figure 2). When nucleotide alignments of VP2 gene parvovirus variants found in this study were contrasted with strain sequences found in other parts of the world, the greater divergence was found between gene sequences of the FPV/ TR/2017/cat3 and the AJ002932.1-FPV-Panocell German strain (92.2% similarity). The FPV/TR/2017/cat3 VP2 sequence had the highest amino acid similarity with most of the international strain sequences used for contrasts, except for the KX943313-FPV-IZSSI Italian strain, which was 99% similar to all field strains found in this study. Indeed, the FPV/TR/2017/cat3 sequence showed high identity with the AY606131.1-FPV French (98.9 %), EU221281.1-FPLV Portuguese (98.9 %), EU498716-FPV 50/07-01-UK (99 %) and KP682520.1-FPV Spanish strains (98.9 %). Further, the high similarity of the FPV/TR/2017/cat3 sequence with FPV strains (which are very close to CPV-2a strains), had a 0.7-0.8% diversity at the nucleotide level with the other obtained Turkish field sequences. The homology rates of the four other analysed strains in this study (FPV/TR/2017/cat1,2,4,5), had a 92.5-99.4% similarity (to sequences of international strains, and are almost identical to each other. These four strains showed the highest amino acid sequence iden tities with the KX943313-FPV-Italy strain (99.4%) and the lowest with the German strain AJ002932.1-FPV-Panocell (92.5%). Similarity rates were 99.1-99.3% with AY606131.1-FPV French, EU221281.1-FPLV Portuguese, EU498716-FPV 50/07-01, UK, and KP682520.1-FPV Spanish isolates. In addition, two of the Portuguese strains (KU248463-FPV-PT015/08 and EU221280-FPLV/cat/39897/PT06) had a discrete sub-cluster with 98.5% identity at the nucleotide level with four of the strains sequenced in this study (FPV/TR/2017/cat1,2,4,5), and a 98.1% identity with the FPV/TR/2017/cat3 strain.
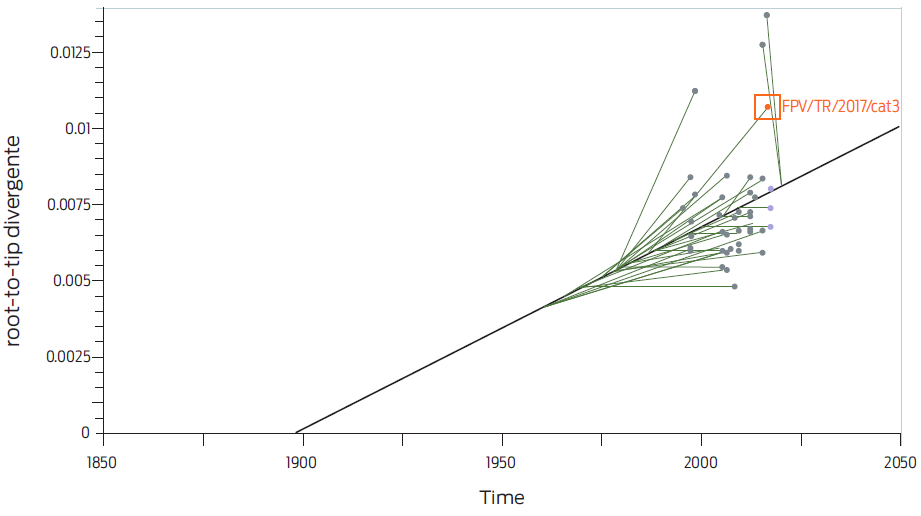
Figure 2 Aminoacid configuration of a relevant gene region of parvoviruses. The points of critical mutation at amino acids 426 and 440 have been highlighted in red.
The Iraqi (KX198140-CPV/Erbil/2016, KX198139-CPV-2/krk/2015) and Italian (KF385391 CPV-2a/Sicily/X29451/2009) strains were localised on a monophyletic clade as a sister branch of CPVs. Moreover, this Italian strain was placed in a distinct paraphyletic branch inside this same group. Both Iraqi strains shared 99% identity at the nucleotide level with each other, and a 99.1% (KX198140-CPV/Erbil/2016) and 99.2% (KX198139-CPV-2/krk/2015) similarity, with the KF385391 CPV-2a/ Sicily/X29451/2009 Italian strain, respectively. Moreover, the Italian strain shared a 98.7% homology with the FPV/TR/2017/cat1,2,4,5 field strains and a 98.2% with the FPV/TR/2017/cat3, sequence. As for the Iraqi strains, the KX198140- CPV/Erbil/2016 showed a 97.8-97.9% and a 97.4% identity with FPV/TR/2017/ cat1,2,4,5 and FPV/TR/2017/cat3, respectively; whilst the KX198139-CPV-2/krk/2015 showed 97.9-98.0% and 97.5% identities with FPV/TR/2017/cat1,2,4,5 and FPV/TR/2017/cat3, respectively. These last percentages of homology with field sequences were similar for the Iraq-Erbil strain.
The MF177229-CPV/368-12-17 strain obtained from Albania, a country close to Turkey, had a 98.2-98.7% homology with the five field strains from this study. Strains JX305950-CPV-2/140/09-33 and FJ222823-CPV-2b-29/97 from Italy (one of the countries closest to Albania) showed 99.8% and 99.6% identity rates respectively, with the Albanian strain. Italian strains JX305950-CPV-2/140/09-33 and FJ222823-CPV-2b-29/97 shared a 98.5-98.6% and a 98.1% homology with FPV/ TR/2017/cat1,2,4,5 and FPV/TR/2017/cat3 strains respectively, which was lower than for the Albanian strain. Nonetheless, the Italian and Albanian strains exhibited almost the same identity with the field strains from this study, at the nucleotide level. A little further west, Spanish strains KP682530-CPV2c/160 and KP682512- CPV/2b/158 had a 99.6% homology with each other and shared a 98.5-98.6% and a 98.1% nucleotide identity with the Italian (JX305950 and FJ222823), FPV/ TR/2017/cat1,2,4,5 and FPV/TR/2017/cat3, strains, respectively. Similarity percent- ages of both Spanish variants with Albanian and Italian strains were 99.6-99.8%. In Portugal, which shares borders with Spain, the KR559891-CPV/2a/PT032/12, KR559892-CPV/2b/PT174/12 and KR559893-CPV/2c/PT178/12 strains were distributed among different clades in the CPV cluster, each representing a distinct CPV subtype. They all shared a 98.0-98.6% nucleotide homology with our five field strains, which was very close to that found for Spanish and Italian strains.
The five strains found in this study had an almost identical homology when compared to the AF469009-MEV/Bergovoy and the KR559893-CPV/2c/PT178/12 Portuguese strains. The highest homology of these two last strains was with the FPV/TR/2017/cat3 sequence. The Russian MEV strain shared a 98.4-98.5% and a 98.2% homology rate with the FPV/TR/2017/cat1,2,4,5 and FPV/TR/2017/cat3 strains, respectively. Field strains from this study and MEV strains were also found to be closely similar. This could be explained by same host recombination events, since minks can be co-infected with MEV and CPV.23 Indeed, there are reports of CPV, FPV and CPV variant (CPV-2a and -2b) recombinations in cats and dogs.5,7
The field strains from this study differed most from the German FPV FJ005200- CPV/G359/97 and FJ005261-CPV/G162/97 CPV strains, that shared a 98.6- 98.7% and a 98.2% identity with the FPV/TR/2017/cat1,2,4,5 and FPV/TR/2017/ cat3 strains, respectively.
In all, the phylogenetic analysis considering 72 international strains with partial VP2 sequences revealed that the FPV/TR/2017/cat1,2,4,5 sequences are clustered together in a monophyletic clade as a branch of CPV-2c, whereas the FPV/TR/2017/cat3 strain fits in a distinct clade, inside CPV-2b. Also, the FPV/TR/2017/ cat3 sequence showed high similarities with the CPV-2b as well as CPV-2a strains. In fact, the FPV/TR/2017/cat3 was the sequence more closely related to the vaccine GU212792 strain.
Amino acid sequence homology between FPV field strains from this study, and between these and several selected parvovirus strains was high. Nonetheless, at position 426, the FPV/TR/2017/cat1,2,3,4,5 field strains were distinct from most parvovirus strains, in that an aspartic acid (D) could be found instead of asparagine (N) (Figure 3). Presence of aspartic acid at position 426 can also be seen in CPV vaccine strains FJ197847/CPVpf, FJ011098/Intervet/vaccine/06, and FJ0110997/Merail/vaccine/06) (Figure 3). Interestingly, variations of amino acids at position 426 impact the antigenic structure of the virus and play an essential role in discriminating CPV-2 variants.24 Also, Threonine (T), which was located at position 440 in almost all of the international strains used for contrasts, was changed to alanine (A) in three of the field strains (FPV/TR/2017/cat1,4,5) from this study. Moreover, the FPV/TR/2017/cat3 strain had two additional substitutions: at position 423, from valine (V) to arginine (R), and at position 425, from T to serine (S), not including other mutations that enable FPV discrimination. Intriguingly, other Turkish aminoacid sequencing analyses regarding local parvovirus strains13 have found N at position 426 and A at position 440, placing all FVP viruses in that study in a monophyletic clade.
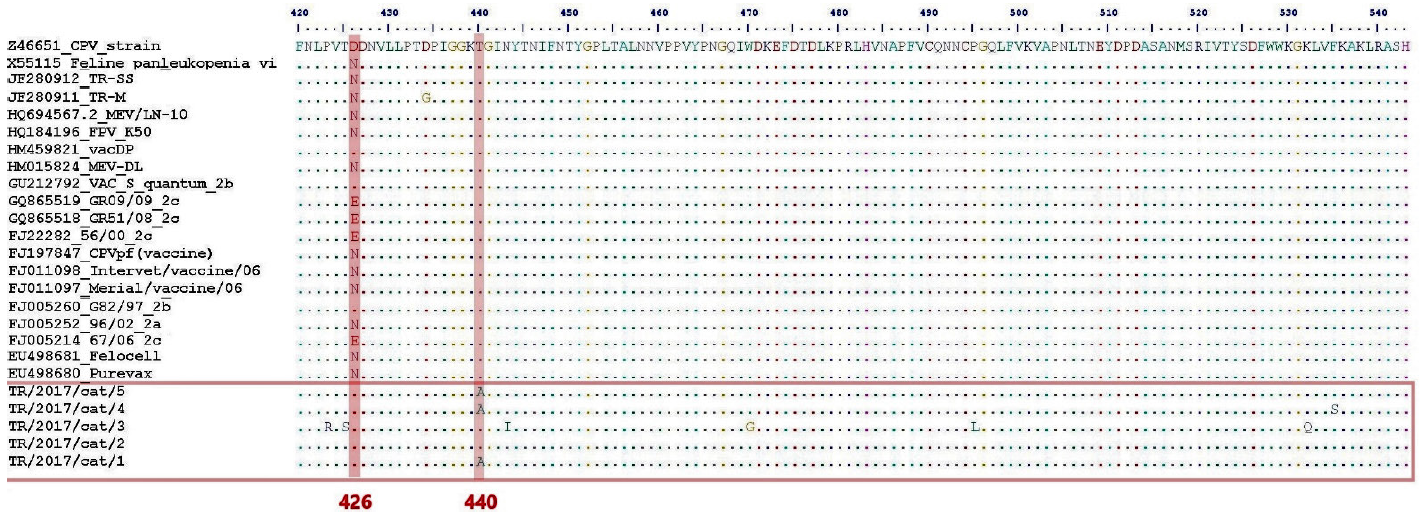
Figure 3 Root-to-tip divergence analysis implemented in TempEst. FPV/TR/2017/cat3 is localized outside the regression line, which indicates a mutational difference.
There are antigenic variants of parvoviruses that can infect multiple hosts.5,12 Recombinations between different CPV antigenic types, as well as between CPV and FPV, are possible.5,7 Diseases caused by each of these virus variants are indistinguishable in carnivores, and combined infections are possible. In particular, the CPV-2 strain is considered a highly pathogenic strain, but less than, and antigenically unrelated to, the CPV type 1 strain.25
To date, there are limited studies describing FPV and CPV gene sequences in Turkish cats, and most examine partial VP2 gene regions.13-15 The present work provides information on the presence and molecular characterisation of FPV field variants of domestic cats in Ankara, specifically by showing differences between full and partial VP2 gene analyses. To our knowledge, this is the first study to examine the nearly full-length sequence of the VP2 gene in Turkey.
The comparison of the complete nucleotide sequences of several CPV-2 and FPV strains with the nearly full-length Turkish VP2 product (1,530 bp) in a phylo-geographic analysis revealed a 0.7-0.8% divergence of the latter from international FPV sequences. In addition, the FPV/TR/2017/cat1,2,4,5 strain sequences shared a 99.8-99.9% identity at the nucleotide level, but FPV/TR/2017/cat3 showed a 0.7-0.8% divergence from the other four field sequences. The present study also exposed a relationship of Turkish FPV with other CPV and FPV international strains, based on partial VP2 analysis. The partial sequences from FPV/TR/2017/ cat1,2,4,5 were found to be clustered together as a branch of CPV-2c, whilst the FPV/TR/2017/cat3 sequence remained distinct in the ML tree and localised close to CPV-2b, clustering with the FPV strains. Partial sequence results differed from the nearly full-length VP2 gene data (Figures 1 and 2), possibly due to the long coding area of VP2, which may increase the possibility of variation.
The FPV/TR/2017/cat3 sequence was found to be closely associated with the GU212792 vaccine strain. Since FPV/TR/2017/cat3 was an outdoor owned animal, adopted from the street, it is not known whether it had been exposed to the virus, if it was in contact with vaccinated animals, or if it was vaccinated against FPV before being adopted. In Turkey, adoption of street and semi-outdoor cats is common. Notably, even when cat 3 and cat 4 lived together, the FPV viruses carried by both animals showed nucleotide differences. For a virus that is resistant to environmental conditions, such as FPV, even minor nucleotide changes are important.
The FPV has strong environmental resistance that facilitates transmission, since it can survive and remain infectious outside the body for prolonged periods.26 Maternal antibodies protect unvaccinated cats from infection for up to three months.3 Thereafter, cats need to be vaccinated to be protected against infection. In Turkey, combined vaccines that include FPV are regularly administered to cats. Prevention is essential to protect animals against infections both from CPV-2b and FPV, as well as to prevent virus spread.27 Canine parvovirus can infect unvaccinated cats, even in animals that carry neutralising antibodies against the FPV virus.28 However, virulence of CPV in animals with FPV antibodies is lower and clinical signs are generally absent, thus the spread of the virus is reduced.28 Nonetheless, Nakamura et al. showed that neutralising antibody titres for CPV-2c were lower than those for FPV29 in cats that were experimentally infected with FPV. Furthermore, differences in virulence between CPV-2 variants can also affect antibody titres.29 Hence, including different CPV-2 variants in vaccines is important, since CPV-2a has been detected in immunized cats against FPV. Moreover, these cats presented with clinical signs.13 In addition, isolation and use of local field variants could be advantageous for adequate prevention and outbreak management strategies. The VP2 region encodes a structural protein of the parvovirus capsid, that is abundant and determines the immune response of the host.2,30 In this study, partial and nearly full-length VP2 gene sequences of 5 local FPV field strains were analyzed. Further studies with a larger number of animals and regions nationwide, to establish FPV strain prevalences are warranted. Data regarding preparation and use of combined vaccines that include local variants, and their potential value, are also necessary. Finally, determination of the antigenicity of strains, the genetic relationship between FPV variants, and their interaction with other strains contained in vaccines could increase the knowledge of disease epidemiology and development of immunity.
Conclusion
This study provides updated information on partial and nearly full-lenght VP2 gene gene sequences of 5 strains of Turkish cat parvoviruses, of which there is limited information. Future studies should include full gene and genome data from different geographical regions in Turkey, to better understand parvovirus transmission in cats that could help develop more effective vaccines.











 text new page (beta)
text new page (beta)



