Introduction
Gastrointestinal nematodes that infect ruminants cause serious health problems that substantially decrease livestock productivity worldwide.1 This condition is further complicated by resistance of parasites against existing anthelmintic drugs.2 Thus, finding alternatives for parasitic control is necessary.
The use of secondary metabolites obtained from legume trees with anthelmintic (AH) properties has become of increasing interest in recent years,3-5 presenting new natural alternatives for sustainable control of parasitic infections.
Reported secondary compounds with anthelmintic properties include condensed tannins, caffeic acid, ferulic acid and flavonoids.6 Moreover, nematicidal activity of flavonoids can be enhanced by their interaction with other compounds such as tannins, which produces a synergic effect.7 Isolated compounds such as caffeoyl and coumaroyl derivatives from a hydro-alcoholic extract of legume Acacia cochliacantha leaves, have also been shown to have a high ovicidal activity (close to 100%) against Haemonchus contortus.8
Plants from the Caesalpiniaceae subfamiliy, including C. coriaria, have analgesic, antibacterial, antifungal and antiparasitic properties.9 The objective of this work was to assess the ovicidal activity of mature fruits and leaves hydro-alcoholic extracts from C. coriaria against H. contortus and H. placei, by an egg-hatching inhibition test.
Materials and methods
Plant material
Mature fruits and leaves were collected from seven Caesalpinia coriaria trees at the site of Palmar Grande, Amatepec in the state of Mexico, Mexico (18° 35’ 6” latitude north and 100° 24’ 59” longitude west, with an altitude of 640 m above sea level). The taxonomic identification of the recovered plant material was performed by an experienced biologist, and a sample was deposited at the herbarium of the Centro de Investigación en Biodiversidad y Conservación from the Universidad Autónoma del Estado de Morelos, Mexico (Voucher code: 35273). Mature fruits and leaves were separately mixed to get a homogeneous sample from all trees, and immedi ately placed in a portable cooler at 4 ºC, for transport to the Laboratory of Animal Nutrition of the University Center UAEM Temascaltepec. Plant material was subsequently left to dry under the shade, until reaching a 100% of dry matter and then ground in a Wiley mill (model 4; Thomas Scientific, Swedesboro, NY, USA), to obtain 2 mm particles.
Hydroalcoholic extracts
To prepare extracts, 200 g of dry matter from either C. coriaria leaves or fruits were independently macerated with a hydro-alcoholic solution (70%, 1:10 ratio, w/v) at room temperature during 24 h. The liquid extracts, free of plant material, were obtained by successive filtration through cotton, four layers of cheesecloth and filter paper (Whatman® 541). Both extracts were then concentrated into a semisolid state, using a rotary evaporator at 50 ºC (R-3 Heidolph, Germany). Finally, extracts were totally dried by lyophilization (Labconco 4.5®) and stored at −40 °C until used for bioassays and phytochemical analyses.
Condensed tannin content
Hydro-alcoholic extracts from fruits and leaves were analysed to quantify total content of condensed tannins (CTC), using the butanol-HCl method.10Lysiloma acapulcensis free condensed tannins (FCT) were used as internal standards.11 Free condensed (FCT1), protein-bound (PCT) and fiber-bound (FCT2) tannins were identified following the technique reported by Porter et al.12 Purification was performed using a Shepadex LH-20 column, as described by Hedqvist et al.13
Identification of secondary compounds by HPLC analysis
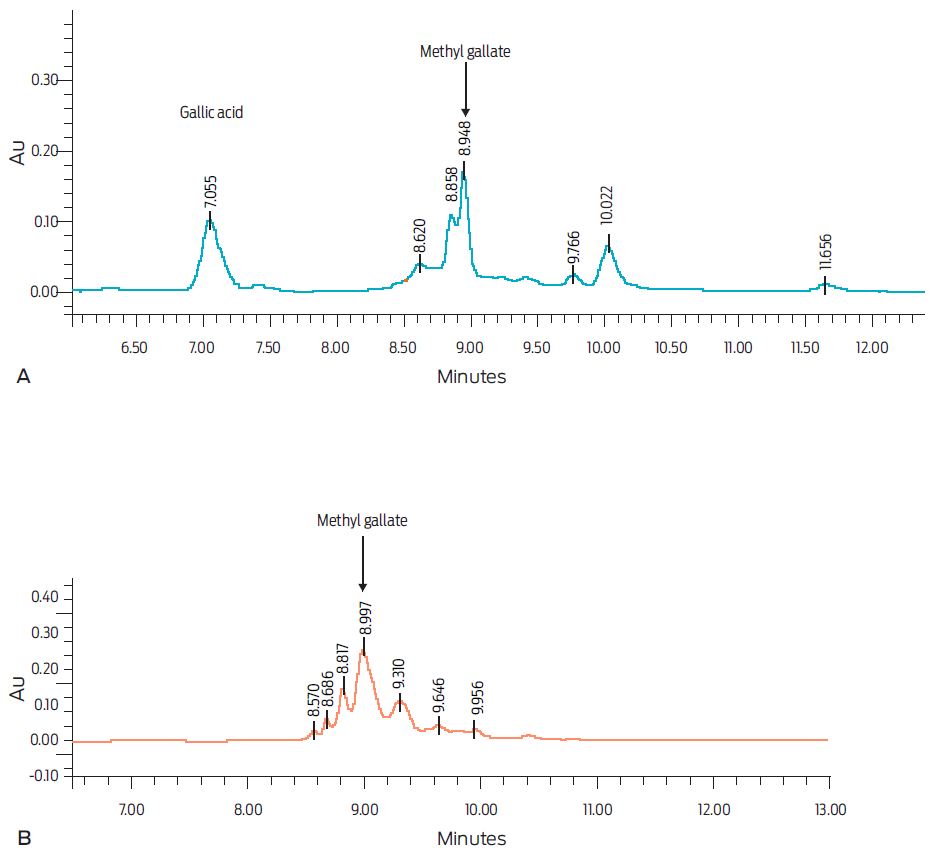
Mature fruits and leaves hydro-alcoholic extracts were analysed using a Waters 2695 separation module HPLC system, equipped with a Waters 996 photodiode array detector and the Empower Pro software (Waters Corporation, USA). Chemical separation was achieved in a supelcosil LC-F column (4.6 mm × 250 mm i.d., 5-µm particle size) (Sigma-Aldrich, Bellefonte, USA). A 0.5% trifluoroacetic acid aqueous solution (Solvent A) and acetonitrile (Solvent B) were used as the mobile phase. The gradient elution was as follows: 0-1 min, 0% B; 2-3 min, 5% B; 4-20 min, 30% B; 21-23 min 50% B; 24-25 min, 80% B; 26-27 min, 100% B; 28-30 min, 0% B. The flow rate was maintained at 0.9 mL/min, and the injection volume was 10 µL. Absorbance was measured at 280 nm. Gallic acid and methyl gallate were identified by contrasting retention times and UV spectra with reference standards (Sigma-Aldrich, St Louis, Mo, USA).14
Parasite eggs
Haemonchus contortus (INIFAP strain) and H. placei (wild strain) eggs were collected from feces obtained from an experimentally infected sheep (16 ± 0.2 kg BW) and calf (175 ± 0.5 kg BW), respectively. To obtain parasite eggs, feces from donor animals were washed, passed through different sieve sizes (200, 100, 72 and 32 µm) and spun with a 40% sucrose solution to separate thicker particles. The resulting material was further washed with tap water, subsequently concentrated by spinning (three times at 3500 rpm for 5 min), and finally re-suspended in sterile distilled water eggs were collected and used immediately in the bioassays.15
Egg hatching inhibition test (EHIT)
Tests were conducted using three replicates and four repetitions in 96-well microtitration plates. Six serial dilutions of both hydro-alcoholic extracts were included: 25.0, 12.5, 6.2, 3.1, 1.5, and 0.0 mg/mL. Distilled water and 0.5 mg/mL of thiabendazole were used as negative and positive controls, respectively. One hundred eggs contained in 50 µl of distilled water and 50 µl of the corresponding extract (or control) were deposited in each well, resulting in a 100 µl final volume per well. Plates were then incubated at 28 ºC with 100% humidity (at an incubator Ecoshell, mod CI-80) for 48 hours. Quantification of non-hatched eggs or larvae was performed using an optical microscope (Motic B3®), in ten-5 µl aliquots placed on a slide per treatment. The egg hatching inhibition percentage was calculated by the following formula: [(number of eggs)/(number of larvae + number of eggs) * 100].15,16
Statistical analysis
Data were analysed by ANOVA.17 A 2 × 2 × 6 completely randomized factorial design was used.18 Analysed factors were: 1) Two types of extract (leaves or fruits), 2) two nematode species (H. contortus and H. placei) and 3) six extract dilution (25.0, 12.5, 6.2, 3.1, 1.5, and 0.0 mg/mL). Mean differences were determined by the Tukey post hoc test (p ≤ 0.05). Finally, the Probit analysis was used to determine effective concentrations (EC50 and EC90) for both extracts, using the SAS program.
Results and discussion
Condensed tannin and other secondary compound content in extracts
The quantity of free, fiber-bound, protein-bound and total condensed tannins for both C. coriaria hydro-alcoholic extracts is shown in Table 1. Polyphenolic compounds in the free-condensed tannin form were the most abundant, with 217.1 g/kg found for the mature fruits extract, and 137.1 g/kg for the leaves extract, in a dry matter (DM) basis. Conversely, protein-bound tannins were present in the lowest amounts (31.7 g/kg, for the mature fruits extract, and 21.7 g/kg for the leaves extract in a DM basis).
Table 1 Free, protein-bound, fiber-bound and total condensed tannin content (g/kg DM) in Caesalpinia coriaria hydro-alcoholic extracts obtained from mature fruits or leaves
| Tannin content | Mature fruit extract | Leaves extract |
|---|---|---|
| Free | 217.1 | 137.1 |
| Protein-bound | 31.7 | 21.7 |
| Fiber-bound | 71.8 | 41.8 |
| Total condensed | 320.6 | 200.6 |
DM: dry matter.
The major compounds identified in both hydro-alcoholic extracts are shown in Figure 1. The chromatographic analysis in the leaves extract revealed the presence of gallic acid and methylgallate (Fig. 1A), whilst only methyl gallate was found in the mature fruits extract (Fig. 1B). García-Hernández et al.19 have previously reported the presence of methyl gallate and gallic acid, as well as other galloyl derivatives, in a hydro-alcoholic extract from C. coriaria fruits. Likewise, Sánchez-Carranza et al.20 found phenolic compounds, including ethyl gallate, gallic acid and tannic acid, in C. coriaria pods.
Egg hatching inhibition
Inhibition of egg hatching in both nematode species increased with greater concentrations of hydro-alcoholic C. coriaria mature fruits or leaves extracts, reaching a 100% inhibition with the 25 mg/mL concentration (p < 0.01) (Table 2). When analyzed independently of concentration or nematode spp., the hydro-alcoholic leaves extract seemed to be more efficient for inhibiting hatching of eggs than the extract from mature fruits (79 and 52% EHI respectively, p < 0.0001). In addition, EHI percentages were higher for H. contortus than for H. placei when either type of extract was added, indicating an apparent increased susceptibility to the hydro-alcoholic extracts for the former species of nematode (76% vs. 55% respectively, p < 0.01).
Table 2 Haemonchus contortus and H. placei egg hatching inhibition test values after incubation with one of two Caesalpinia coriaria hydro-alcoholic extracts (from fruits or leaves) for 48 h
| Treatments | Egg hatching inhibition (%) | |
|---|---|---|
| Haemonchus contortus | Haemonchus placei | |
| Mature fruits extract (mg/mL) | ||
| 25.0 | 100a | 100a |
| 12.5 | 100a | 100a |
| 6.2 | 99.5a | 92.75a |
| 3.1 | 89.25a | 33.75cd |
| 1.5 | 58.75b | 17.75e |
| 0.0 | 3.25fg | 2.75g |
| Leaves extract | ||
| 25.0 | 95.25a | 92.50a |
| 12.5 | 90.00a | 60.75b |
| 6.2 | 60.00b | 22.75de |
| 3.1 | 40.75c | 19.75de |
| 1.5 | 27.50cde | 16.25efg |
| 0.0 | 3.25fg | 2.75g |
| Thiabendazole (0.5 mg/mL) | 100a | 100a |
| P-value (Extract*Concentration*Nematode) | <0.0001 | |
| SEM | 0.10 | |
Means with different letters represent significant differences p < 0.05.
Haemonchus contortus and H. placei have a high prevalence as gastrointestinal nematodes in small ruminants and cattle under tropical grazing conditions.21,22
This work shows evidence of the ovicidal effect of two C. coriaria tree extracts against both parasites. Plants belonging to Caesalpiniaceae subfamily have been shown to possess different medicinal properties. Anthelmintic effects have been particularly found in the Caesalpinia genus.23,24 These results coincide with those recorded in the present study, and could be related to the content of free condensed tannins, which is higher than in other leguminous species from the same region, such as Lysiloma acapulcensis11 and Acacia cochliacantha.4,8
Phenols, such as tannins, may negatively impact nematode egg hatching by binding to the egg cuticle which is rich in glycoproteins, forming a tannin-protein complex that may alter embryo development and eventually the hatching process.25,26 Parasitic load in H. contortus infected hair sheep was decreased when animals were fed with leaves of the L. acapulcensis tree, with an added beneficial effect in productive performance.27 Authors found a high content of condensed tannins (116 g/kg of dry matter) in these leaves. Both C. coriaria extracts (leaves and mature fruits) used in this study had a higher content of condensed tannins than that found for L. acapulcensis.
The effective concentrations EC50 and EC90 obtained with both C. coriaria mature fruits and leaves extracts are shown in Figures 2 and 3, respectively. The EC50 and EC90 for the C. coriaria fruits extract were: 1.63 and 3.64 mg/mL respectively, for H. contortus eggs, and 3.91 and 6.05 mg/mL respectively, for H. placei eggs. Effective concentrations using leave extracts were: EC50 > 3.98 and EC90 > 16.61 mg/mL for H. contortus eggs, and EC50 > 11.68 and EC90 > 24.07 mg/mL, for H. placei eggs (Fig. 3).
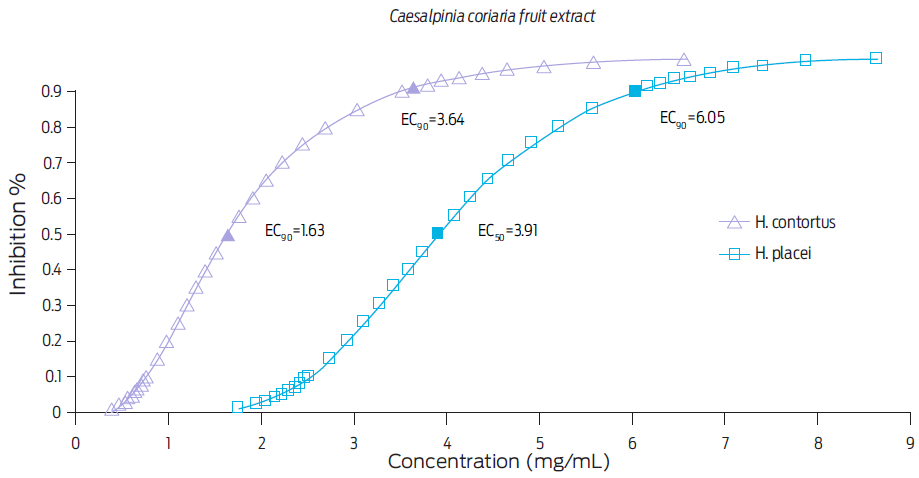
Figure 2 Effective concentrations (EC50 and EC90) required to inhibit hatching of H. contortus and H. placei eggs after a 48 h incubation with a Caesalpinia coriaria mature fruits extract, as determined by Probit analysis.
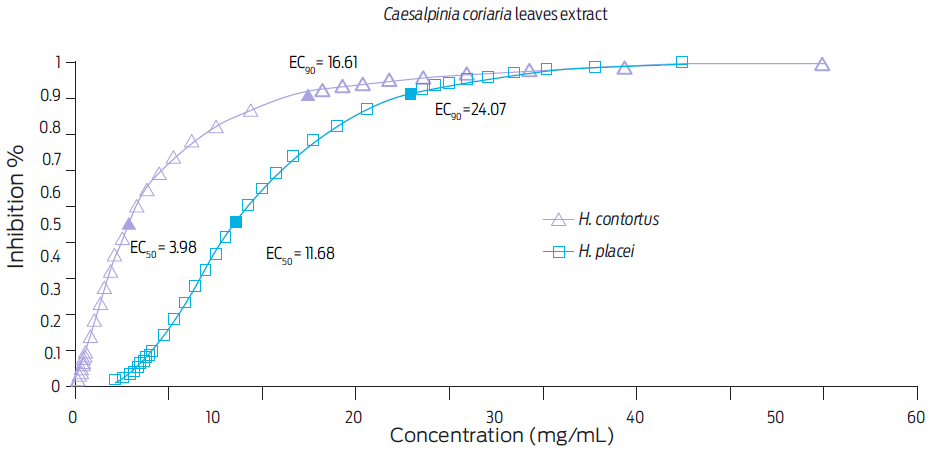
Figure 3 Effective concentrations (EC50 and EC90) required to inhibit hatching of H. contortus and H. placei eggs after a 48 h incubation with a Caesalpinia coriaria leaves extract, as determined by Probit analysis
A stronger ovicidal effect was observed when the fruits extract was used on both parasite species. In fact, the EC90 value for the fruits extract was fivefold than that obtained when the leaf extract was used on H. contortus eggs or fourfold on H. placei eggs. Differences for effective concentrations found for leaves and fruits extracts could be associated with content of phenolic compounds.7 A report of a 93% reduction in the number of Trichostrongylus eggs per gram of feces of infected sheep, was recorded when a methanolic extract from a leguminous of the same genus (Caesalpinia crista) than the plant used for this study, was administered orally (3 g/kg live weight). This same study found an EC50 of 0.134 mg/mL through the EHIT.28
Other specific compounds derived from secondary metabolism of Caesalpinia spp. leaves, such as ellagocatechins and gallocatechins,28,29 have also been found to have nematicidal effects.29-31 Both gallic acid and methyl gallate, which were found in extracts used in the present study, are considered as the basic chemical structure of hydrolysable tannins.32 The nematicidal activity of different plants has also been attributed to these two compounds.7 Indeed, a recent study by De Jesús-Martínez et al.,33 found a high ovicidal activity of a methanolic extract of C. coriaria fruits harvested in Guerrero, Mexico, and the anthelmintic effect was associated with gallotannin content (methyl gallate). Similarly, another study showed that gallic acid and other galloyl derivatives obtained from C. coriaria fruits were associated with hatching inhibition of five gastrointestinal parasitic nematode eggs (Haemonchus spp., Cooperia spp., Ostertagia spp., Trichostrongylus spp. and Oesophagostomum spp.). However, these authors also showed that methyl gallate had no activity against these parasites.19 Therefore, the increased efficiency in ovicidal activity (against both parasites species) observed for the hydro-alcoholic ex- tract from C. coriaria leaves in the present study could be associated with its gallic acid content.
The anthelmintic activity of condensed and hydrolysable tannins has also been documented.34,35 To ascertain the anthelmintic effect of condensed tannins in particular, addition of polyethylene glycol (PEG) to the tests is imperative, since PEG has the ability to bind and inactivate tannins.36,37 Since PEG was not used in this study, the observed ovicidal effect of C. coriaria extracts cannot be directly attributed to their condensed tannin content, but it could be associated with found gallotannins.19 Hence, further in vitro studies are warranted (using PEG) to verify if condensed tannin content in extracts from C. coriaria fruits and leaves does in fact have an ovicidal activity.
Other studies ascribe nematicidal activity to free condensed tannins. For in- stance, Williams et al.6 evaluated acetone extracts of diverse tanniniferous plants (Ribes nigrum, Tillia cordata, T. vulgaris, Salix spp. and Triflorium repens) by the determination of EHT and larval development (L3) of Oesophagostomum dentatum (a pig nematode), and found a 90% inhibition of larval development at a 125-µg/mL concentration of all extracts used. The ovicidal effect of the studied extracts could also be related to the combined effect of phenols such as methyl gallate, gallic acid and flavonoids, which could interfere with the processes of embryo development of the larva within the egg. Indeed, ultrastructural damage on the cuticle of Cooperia spp. eggs has been observed through transmission electronic microscopy, after being exposed to phenols (quercetin and caffeic acid) isolated from L. leucocephala leaves.37 On the other hand, Brunet et al.30 observed damage of internal and external structures of infective larvae of H. contortus and Trichostrongylus colubriformis (such as structural changes in the hypodermis, cytoplasmic vesicles and muscular and intestinal cell degeneration), after a 3 h contact with Onobrychis viciifolia condensed tannins.
Genetic diversity of each nematode parasite could also affect the efficiency of the ovicidal activity of fruits and leaves extracts from C. coriaria. In their adult stage, both nematode species can be distinguished by their morphological structures such as the spicules and copulatory bursa in males, or the synlophe in males and females.38 A tri-layer complex cuticule has been described for H. contortus eggs, with the external and middle layers composed of protein fibrils and chitin, respectively, as well as a semipermeable internal layer composed of lipids and glycoproteins.39 Information for H. placei egg composition is however very limited. The greater ovicidal activity found for H. contortus when compared to H. placei, independently of the type of extract used in this study could therefore be due to structural biochemical or molecular differences between these parasites. Future studies to establish and identify these factors are warranted.











 nueva página del texto (beta)
nueva página del texto (beta)