Introduction
Pregnancy-associated glycoproteins (PAGs) belong to the family of inactive aspartic proteinases.1 When first identified, they were known as pregnancy-specific protein B (PSPB),2 pregnancy-specific protein 60 (PSB-60)3 and Sheep Biology Unit-3 antigen (SBU).4 PAGs were definitely named by Zoli et al( 5 after being isolated from bovine placentae, by means of a similar approach to that described by Butler et al2 These glycoproteins are produced by ruminant trophoblasts and released into the intercotyledonary space, also reaching maternal circulation. PAGs are produced from the stage of embryo implantation and continue throughout gestation.6,7 In fact, PAGs are thought to be involved in maternal recognition of pregnancy.8 Presence of PAGs in maternal circulation allows for an early diagnosis of pregnancy,9,10 detection of late embryonic or early fetal deaths, identification of twin pregnancies, and monitoring of placental function.11,12 Also, early detection of non pregnant animals permitsa prompt reintoduction to appropriate reproductive management practices thereby reducing the calving interval. PAGs can be used for early diagnosis of pregnancy between days 26-30 post-insemination, since concentrations in maternal blood prior to this particular interval are highly variable and can thus jeopardize accuracy of test results.13 Factors that can affect serum PAGs concentrations are day of gestation, fetal number sire, milk production and season.14-16 To the authors’ knowledge, the impact of different synchronization protocols on serum PAGs levels has not been investigated.
Development of synchronization protocols ensues a low efficiency of estrus behavior detection in large-scale enterprises.17 In dairy cattle, these protocols can include synchronization of oestrus and/ or of ovulation. Designed protocols are based on either shortening the luteal phase (via lysis of the corpus luteum with Prostaglandin F2α [PGF2α] or its synthetic analogues), or lengthening diestrus (by use of exogenous progesterone or progestagens).17 When synchronization of ovulation is sought, both gonadotropin-releasing hormone (GnRH) and prostaglandins are used. At present, there are several available GnRH based timed artificial insemination (TAI) protocols,18 the most commonly used being the ovsynch protocol.19 Reported pregnancy rates after implementation of an ovsynch protocol appear to be similar to those achieved after PGF estrus synchronization.20,21 However, fertility rates can be lower due to poor synchronization of ovulation in approximately one-third of ovsynch-treated cows18, and to ovulation of immature follicles.22,23 Moreover, GnRH-based synchronization protocols have been associated with lower pregnancy and higher embryonic death rates.18,23 This may relate to the stage of the oestrous cycle at which the GnRH-based protocol is initiated.24,25 Indeed, when GnRH was administered to cattle between days 1-4 or 14-21 of the estrous cycle, pregnancy rates were found to be lower than when given between days 5 and 13.24 In fact, when GnRH is administered during metestrus (Days 1 to 3), the dominant follicle may not ovulate, and hence undergo atresia at the established time for PGF injection. As for GnRH administration on days 13 to 17 of the estrous cycle, the dominant follicle of the second wave may also not ovulate, and luteolysis could result from endogenous PGF release, with a consequent early asynchronous ovulation regarding TAI, which results in low pregnancy rates. Moreover, Perry et al23 reported that GnRH-induced ovulation of physiologically immature follicles (< 11 mm in diameter) was associated with lower blood estradiol levels on the day of insemination and a lower rate of increase of progesterone concentrations after insemination. However, follicle diameter has been shown to have no effect on fertility when the follicle is physiologically mature.23
Progesterone plays a critical role in conception, embryonic development, and pregnancy maintenance in cattle.26 In vitro research has shown that progesterone output is increased when PAGs are added to luteal cell cultures.27,28 Moreover, a positive correlation has been shown between serum level of PAGs and progesterone concentrations in dairy cattle,29,30 sheep,31 and goats.32 Since low blood progesterone concentrations are associated with GnRH-based protocols, we hypothesized that serum level of PAGs would also be low in ovsynch-treated heifers. Therefore, the present study aimed to assess 1) Any correlation between serum progesterone and PAGs concentrations at day 28 post-insemination, and 2) the effect of different synchronization methods on serum progesterone and PAGs concentrations on day 28 of pregnancy in dairy heifers.
Materials and methods
Animals and experimental design
This work was carried out between November 2016 and February 2017, at the Ceylanpinar Agricultural Enterprise in Turkey. The use of animals was approved by the Local Ethics and Animal Welfare Committee for Animal Research of DOLL-VET.
For this study, 210 reproductively sound and clinically healthy 15 to 20-month old Holstein heifers were used. All animals weighed between 340 and 400 kg and had a body condition score of 3.0-3.5. Heifers were kept in a semi-enclosed stall housing system, fed on a total mixed ration (TMR) (dry matter 90%, metabolizable energy 2550 kcal/kg, crude protein 16%) and given ad libitum water. Vaccination against reproductive pathogens and treatment for internal and external parasites were administered at least 2 months prior to the set date for artificial insemination.
Synchronization protocols were started in all animals without previously establishing the stage of the estrous cycle. The presence of pre-ovulatory follicles and the lack of a corpus luteum were determined by rectal palpation and ultrasonography in heifers selected for AI. All inseminations were performed with proven fertile semen (obtained from the laboratory of the General Directorate of Agricultural Enterprises TİGEM, of the Ministry of Agriculture and Forestry), by an experienced veterinarian.
Heifers were randomnly allocated to three groups: Animals from the Control group (n = 70) did not receive any hormonal treatment, and were monitored for signs of estrous 2 to 3 times daily, for at least half an hour, for a 21-day period. Fifty heifers showing standing behavior, which was considered as the definite sign of estrus, were inseminated in this group. Animals in the Ovsynch group (n = 70), were synchronized with the Ovsynch protocol.19 Namely, 100 µg of gonadorelin diacetate tetrahydrate (GnRH, Ovarelin®, Ceva Hayvan Sağlığı, Istanbul, Turkey) were administered to heifers by intramuscular (im) injection at the start of treatment (day 0), followed by 500 µg of im cloprostenol sodium (PGF2α, Juramate®, Ege Vet Hayvancılık, Izmir, Turkey) on day 7, and a second 100 µg GnRH injection on day 9. Fixed-time artificial insemination was performed sixteen hours after treatment completion, only in heifers that had a preovulatory follicle and lacked a CL, confirmed by examination of the ovaries (n = 52). Heifers in the PG group (n = 70) received two injections 500 µg of PGF2α 11 days apart. Animals were observed for oestrus behaviour 2-5 days after the second PGF2α injection, 2 to 3 times a day, for at least half an hour. Heifers showing signs of oestrus were artificially inseminated at the time of heat detection (n = 45).
Blood sample collection
Blood samples were collected from the coccygeal vein into dry vacuum tubes on day 28 post-AI. Samples were centrifuged at 3000 rpm for 15 minutes for serum extraction, which was subsequently stored at −20°C until assayed.
Pregnancy diagnosis
Pregnacy was diagnosed through identification of the embryonic vesicle and of the fetal heartbeat by transrectal ultrasonography (Hasvet 838) at day 28 post-AI. Maintenance of pregnancy was subsequently confirmed by rectal palpation, through verification of slipping fetal membranes on days 60 and 90, and presence of placentomes or the foetus on day 120.
Measurement of serum PAGs and progesterone concentrations
Serum PAGs concentrations were measured using a commercial ELISA kit (IDEXX Laboratories, Inc., Westbrook, ME, USA), at the laboratory of the distributor of the manufacturer in Turkey (Diagen Corp.), by a skilled technician and in accordance with the manufacturer’s instructions. Briefly, an anti-PAG monoclonal antibody was coated onto a microtiter plate for the configuration of a plate format. This monoclonal antibody was produced against the PAG-55 protein fraction, which is composed of PAG-4, PAG-6, PAG-9, PAG-16, PAG-18, and PAG-19.33 Once the diluted sample was incubated within the coated well, captured PAGs were detected with the aid of PAGs-specific antibodies (detector solution) and the horseradish peroxidase conjugate. After the unbound conjugate was washed away, 3,3’, 5,5’-tetramethylbenzidine substrate was added to the wells. Colour reaction was measured with a spectrophotometer at a 450 nm wavelength. Result calculations were based on the optical density (OD minus background) of the samples. PAGs concentrations in a sample were determined by subtracting the mean OD value of the negative controls from the OD value obtained for each specific sample (S-N value), and proportionally relating the colour intensity to the amount of PAGs in the samples. There were two negative and two positive controls in each plate.16 Particularly, for pregnancy diagnosis through the PAGs assay, when the S-N value was ≥ 0.300 the samples were deemed as positive (pregnant), and when the S-N value was below 0.300 the samples were considered negative (nonpregnant).34
Serum progesterone concentrations were determined by a commercial bovine progesterone ELISA test kit (Wuhan Fine Biological Technology Co., Ltd., China), in accordance with the manufacturer’s instructions.
Statistical analyses
Differences in PAG and progesterone concentrations between groups were analyzed by one-way ANOVA and the Tukey’s post-hoc test, using the SPSS® (SPSS 18.0, Chicago, IL, USA) software package. Normality of data was assessed by the Shapiro-Wilk’s method. Results are expressed as mean ± standard error (SE) of the mean. PAGs and progesterone correlations were determined by the Pearson’s test. Pregnancy rates, conception rates and fetal death rates were analyzed by the chi-square test. Statistical significance was set at p < 0.05. Pregnancy rate was defined as the percentage of pregnant animals from the total number of heifers within a group. The conception rate was considered as the percentage of animals determined to have conceived, from those that were artificially inseminated.
A positive diagnosis of pregnancy by transrectal ultrasonography was the reference test to which serum PAG test results were compared. The diagnostic value of the PAG ELISA used in this study was determined by establishing false positive(a), false negative (b), true positive (c) and true negative (d) results; and by subsequently calculating the sensitivity [a/(a+c)X100], specificity [d/(b+d)X100], positive predictive value (PPV) [a/(a+b)X100], negative predictive value (NPV) [d/(d+c)X100], and accuracy of the test [(a+d)/(a+b+d+c)X100].
Results and discussion
Conception and pregnancy rates
Heifers in the control and PG groups were considered to be in estrus when displaying the distinctive standing behaviour. Estrus was detected in 71.4% (50/70) and in 64.2% (45/70) of the control and the PG group heifers respectively. All animals in the control and PG groups detected in heat were artificially inseminated, after the presence of a preovulatory follicle and the absence of a corpus luteum were confirmed by rectal palpation and ultrasonography of the ovaries. Verification of these same ovarian characteristics was necessary to consider that a heifer from the ovsynch group positively responded to the synchronization protocol. Following this confirmation, 74.3% (52/70) of the animals in this last group were inseminated 16h after treatment completion. Accordingly, Colazo et al35 reported that 11% of the cattle ovulated before TAI and 15% did not respond to PGF injection after using an ovsynch protocol for estrus synchronization.
Conception (Control 50% Ovsynch 42% and PG 47%) and pregnancy (Control 36%, Ovsynch 31% and PG 30%) rates determined on day 28 post-AI, did not differ between groups (p > 0.05; Table 1).
Table 1 Pregnancy and conception rates of non-synchronized and synchronized heifers on Day 28 post-AI.
| Group | N | Number of artificially inseminated animals | Number of pregnant animals | Pregnancy rate* % | Conception rat e** % |
| Control | 70 | 50 | 25 | 36 | 50 |
| Ovsynch | 70 | 52 | 22 | 31 | 42 |
| PG | 70 | 45 | 21 | 30 | 47 |
| P value | > 0.05 | > 0.05 |
* Pregnancy rate: pregnant heifers/total number of heifers within a group.
**Conception rate: pregnant heifers/artificially inseminated heifers.
There are reports of similar conception and pregnancy rates after natural breeding or AI in synchronized cows using either a double injection of PGF 2α or the ovsynch protocol.20,36-38 However, lower percentages of both parameters have been reported after natural presentation or synchronization of estrus in cattle, due to inefficient heat detection or early embryo deaths.39 Hence, the lack of difference found for conception or pregnancy rates between groups in this study may have resulted from an absence of impact of the synchronization protocols on reproductive parameters, or it could derive from a decreased statistical power due to the low number of included observations.
Serum PAGs and Progesterone concentrations
PAGs concentrations (OD) were found to be different between pregnant (3.31 ± 0.07) and non-pregnant (0.12 ± 0.02) heifers (p < 0.001). However, no differences were found between treatment groups in pregnant animals (Control 3.37 ± 0.15, n = 25; Ovsynch 3.21 ± 0.14 n = 22 and PG 3.34 ± 0.15, n = 21) (p > 0.05). Other studies have also found similar PAGs concentrations in dairy heifers during the 4th week of gestation using the same commercial PAG ELISA test kit.34
Serum progesterone concentrations of the pregnant heifers in the Ovsynch Group were lower (4.70 ± 0.17 ng/mL) than those found in the control (5.37 ± 0.08 ng/mL), or the PG (5.34 ± 0.13 ng/mL) groups (p < 0.001; Figure 1). There are reports that suggest that GnRH-based protocols are associated with low blood progesterone concentrations.23 Nevertheless, the lower levels of progesterone found for the Ovsynch group in this study fell within established values for early gestation in cattle(40, and did not have an impact on conception, pregnancy or fetal death rates.
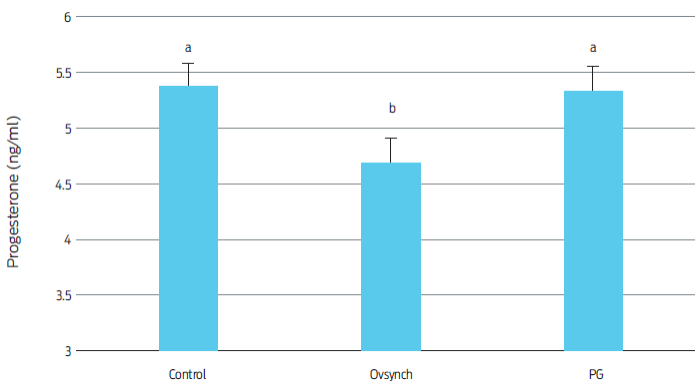
Figure 1 Mean progesterone concentrations on control and synchronized heifers on day 28 of pregnancy. a,bDifferent superscripts indicate differences between groups (p < 0.001).
No correlation was found between mean serum PAGs and progesterone concentrations on day 28 post-AI (r = 0.024, p = 0.85). While some authors state that blood PAGs and progesterone concentrations are not correlated,14,15,41 others suggest that PAGs levels may positively impact serum progesterone.29-32 In fact, in vitro research has revealed that the administration of PAGs to luteal cell cultures may increase progesterone output by these cells.27,28 Conversely, above average progesterone concentrations on day 21 of gestation have also been related to an increased PAGs level through the first trimester of pregnancy in dairy cattle.29 The correlation between PAGs and progesterone was assessed in the present study using relatively few samples, which were taken at a single time point. Further research that considers multiple sampling time points and a higher number of animals may yield more accurate results.
Diagnostic characteristics of the serum PAG-ELISA test
The PAGs ELISA test was used to distinguish between pregnant and non-pregnant animals and to calculate relative PAGs concentrations in sera of inseminated heifers. Values for sensitivity, specificity, PPV, NPV and accuracy of the test on day 28 post-IA were 100%, 94,9%, 94,4%, 100% and 97,3%, respectively. Our test results agree with those obtained in previously conducted studies that used a similar assay in the 4th and 5th weeks of cattle pregnancy.16,34,42
Embryo and fetal death rates
Reproductive performance of a cattle herd can be heavily impacted by embryo and fetal deaths. Both embryonic and fetal death rates have been found to increase when GnRH-based estrus synchronization protocols are used in cattle.22,23 In the present study, there were no embryo or fetal deaths at days 28, 60 or 90 of pregnancy. However, examinations for pregnancy confirmation at day 120 revealed two fetal losses in the control group (8%), one in the Ovsynch group (4.5%), and one in the PG group (4.8%). The overall rate of fetal death at this time point was 5.9% with no differences found between groups (p > 0.05).
Low plasma PAGs concentrations during early pregnancy have been related to early embryonic and late fetal deaths in cows.16,43-47 The mean serum PAGs and progesterone concentrations on day 28 in heifers that lost their calf at day 120 of pregnancy in this study were 3.79 ± 0.19 and 5.53 ± 0.21 ng/ml, respectively, which were similar to obtained values for heifers that completed their pregnancies (3.24 ± 0.09 and 5.11 ± 0.09 ng/ml, respectively; p > 0.05).
Conclusion
Serum progesterone concentrations in early pregnancy were found to be lower in synchronized dairy heifers with the Ovsynch protocol. However, no differences were observed between treatment groups regarding serum PAGs concentrations at day 28 of gestation. Furthermore, no correlation was detected between serum PAGs and progesterone levels, or between PAGs concentrations in early pregnancy and fetal deaths on day 120 of pregnancy. Further research including multiple sampling time points throughout gestation is warranted to determine changes in PAGs and progesterone profiles, their temporal correlation, and if there is a related impact on embryo or fetal death in dairy heifers.











 text new page (beta)
text new page (beta)



