Introduction
Dogs have had a close and positive relationship with humans for approximately 15,000 years.1,2 However, worldwide canine overpopulation has increasingly turned into a serious public health problem, particularly in underdeveloped countries.3,4
Several programs and strategies have been implemented to control this problem. However, these have mostly been insufficient or unsuccessful, due to attached economic, ethical and/or social constraints.5,6 Thus, there is a need to continue searching for adequate alternatives to control canine reproduction.
The bitch is the only domestic animal that has an anestrus stage considered within the estrous cycle, which lasts between two and six months. During early and mid-anestrus, the hypothalamus and ovaries of bitches are relatively inactive. However, this condition changes during late anestrus, when there is an increase in expression of genes encoding for estrogen receptors, and for the P450 aromatase enzyme, which enhance estrogen biosynthesis in preparation for a new estrous cycle.7 Both estradiol (E2) and progesterone (P4) have fundamental roles in periodicity of the estrous cycles in bitches.8
Coumestrol (COU) molecules can bind to and activate estrogenic receptors (ER) α and β, thus mimicking estrogenic actions. Administration of COU has been reported to enhance proliferation of uterine and vaginal epithelia, as well as to reduce fertility in mares, cows, female bats, ewes, and rats.9-14
In mice, bitches, and male dogs, use of COU has been assessed as a treatment for controlling reproduction.15-17 COU is commonly diluted in dimethyl sulfoxide (DMSO) before administration. However, DMSO may independently influence reproduction in mammals, since it has been shown to modulate estrogenic responses in hepatocytes of fish,18,19 and to induce cell differentiation of rat mammary glands.20 Indeed, previous results from our lab show that COU and/or DMSO are estrogenic when administered to periovulatory stage bitches.15 In fact, peripheral levels of both P4 and E2 were modified, and numbers of cornified vaginal cells were increased. Nonetheless, to the best of our knowledge, there have been no studies where administration of COU and/or DMSO during a non-estrogenically susceptible stage (such as anestrus in bitches) has been assessed.
Thus, our hypothesis was that both COU and DMSO would increase circulating E2 levels, decrease peripheral P4, and alter vaginal cell types in anestrus bitches, without affecting diestrus or anestrus lengths. Therefore, our first aim was to evaluate the effects of a single oral administration of COU diluted in DMSO or of DMSO-alone, on the above-mentioned variables, whilst a second objective was to clinically assess the effects of these treatments on health status of experimental dogs.
Our findings show for the first time that DMSO by itself has estrogenic effects in anestrus bitches. Therefore, results of earlier studies where estrogenic actions were attributed to COU when diluted in DMSO should be re-examined.
Materials and methods
Ethical review
All procedures complied with the Mexican Official Regulation NOM-062-ZOO-1999, under protocol number DC-2015 / 2-10 for the Production, Care and Use of Laboratory Animals. A written consent was provided by dog owners for the inclusion of their animals in this research.
Animals and general management
Fifteen sexually mature bitches, which remained at their owner’s house throughout the study, were used. Individual animals were given different brands of commercial dry dog food (kibbles), occasionally complemented with household meal leftovers. Bitches also had free access to water. Housing and general care of animals were deemed adequate by researcher inspection prior to the start of the trial. Bitches had health cards and a history of regular visits to a veterinary center. Proper internal and external parasite control, as well as vaccination against leptospira, distemper, adenovirus, parvovirus, parainfluenza, and rabies were confirmed. In addition, a clinical visit by a qualified veterinarian was scheduled at the beginning of the trial to establish an adequate health status. A complete blood cell count test was also used to evaluate overall health of bitches. In addition, animals had not been diagnosed with any disease or received any antibacterial medication during the six months preceding the beginning of the study. Fifteen sexually mature (4.53 ± 1.80 years old) intact bitches that had been in estrus at least once were used. Animals weighed 8 ± 4.29 kg and were all from small-to-medium size breeds (5 Poodles; 3 Schnauzers; 2 Pugs; 1 Yorkshire; 4 mixed-breed dogs). Bitches were in anestrus at the beginning of the experiment, as determined by waiting for a 2-3 months period subsequent to estrus detection. In addition, a vaginal smear (EVC) was taken to confirm a cell pattern typical to anestrus.22
Treatments
Animals were randomly assigned to one of the following treatments: 1) Control group (n=5), in which bitches received a single commercial dog food biscuit (Pedigree®) with no additives at the beginning of the study (day 0); 2) COU-treated group (n=5), where animals received a single biscuit instilled with COU (600 µg/kg of body weight; Sigma Chemical Co. St. Louis, Mo, USA) which was diluted with DMSO (20 µL per 600 µg of COU; Sigma Chemical Co. St. Louis, Mo, USA, 99.9% purity); and 3) DMSO-treated group (n=5), where a biscuit that contained only DMSO (20 µL/kg of body weight) was given to bitches. All animals were weighed prior to treatment delivery, and appropriate doses for each individual were infused to the center of a biscuit by means of a 10-100 µL micropipette. Biscuits were left for between 10 and 15 min after drug infusion, to ensure compounds were well absorbed prior to delivery. Complete ingestion of biscuits was also verified. The chosen dose of COU had been previously tested in male dogs.23 DMSO quantity (20 µL) was set at the minimum required for adequate dilution of 600 µg of COU (without the need of vortexing or heating), that had been approved by the Food and Drug Administration (FDA) for dogs.24
Biological samples
Hormones
Blood was drawn from cephalic veins on days 0, 14, 21, and 28 post-treatment, as well as every 30 days for an additional 6-month period. P4 and E2 were measured in serum by immune-enzymatic assays (Enzyme-Linked Immune-Absorbent Assay, DGR Instruments, GmbH, Germany). The assay curve for E2 and for P4comprised values from 10.6 to 2000 pg/mL and 0.01 to 40 ng/mL respectively.
EVC
A vaginal smear was taken from the caudal portion of the vagina on days 0, 7, 14, 21 and 28. Cells were transferred by rolling the swab onto a glass slide, fixed with Citospray (CTR Scientific, Mexico), and stained using a modified Papanicolaou procedure (by using saline solution instead of distilled water), to preserve erythrocytes.25 Parabasal, intermediate, and superficial (nucleated and anucleated) vaginal cells were counted by optical microscopy with a 40x objective (one randomly chosen slide per animal; Axio Scope.A1, Zeiss, Mexico).
Anestrus and diestrus duration
Owners were taught to identify and then report signs of estrous. Beginning of diestrus was retrospectively established as the day following disappearance of estrus signs with P4 levels over 2.5 ng/mL. The end of diestrus was established as the day when P4 had fallen under 2.5ng/mL in at least two consecutive samples. The 2.5 ng/mL point between diestrus and anestrus was set from a maximum previously observed P4 level of 2 ng/mL in anestrus bitches26. Assay sensitivity for P4 assays allowed to statistically distinguish 2.0 ng/mL from 2.5 ng/mL in standard curves (P<0.05, regression analysis). Beginning of anestrus was established as the day following end of diestrus.
Design and statistical analysis
Serum E2 and P4 levels and vaginal cell counts for the first 28 days of the study, as well as hormone concentrations between months 2 and 6 were analyzed by ANOVA in a completely randomized design for repeated measures (PROC GLM, SAS, version 9.3; SAS Ins. Inc., Cary, NC, USA). The statistical model included the effects of treatment, animal nested within treatment, day, and treatment by day. The Tukey-Kramer test was used for post-hoc mean contrasts. Length of anestrus and diestrus was analyzed by ANOVA (GLM,SPSS Statistics, version 24, IBM, USA), and the post-hoc Bonferroni test.
Results and discussion
Hormones
Levels of serum P4 remained constant during the first 28 days post-treatment in control and COU groups (Figure 1A). This result is in contrast to a previous work15 where administration of COU significantly reduced P4 on days 21 and 28 post-treatment in periovulatory stage bitches. However, serum P4 levels found in this study were basal to begin with, which could explain this difference. Alternatively, COU effects on P4 may be stage-dependent. To our knowledge, there have been no other published studies where COU had been given to bitches, however, stage-dependant effect of COU have been reported in other animal models. Indeed, addition of COU to luteal cell cultures did not affect P4 secretion27 in samples from pregnant cows, but levels were increased on samples from days 16-19 of the estrous cycle, or decreased on luteal cells from days 11-15.28
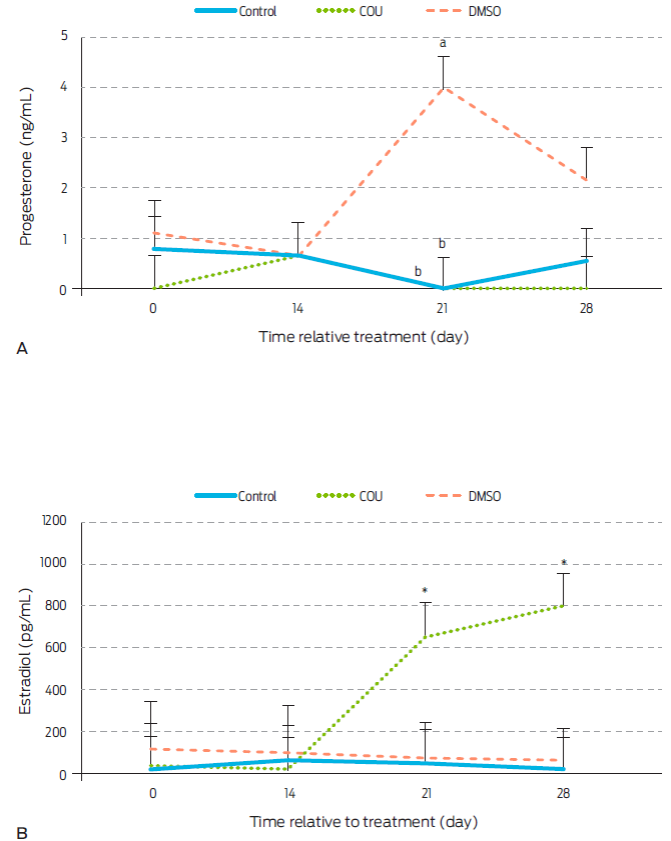
Figure 1 Progesterone (A) and estradiol (B) serum levels in anoestrous bitches receiving a commercial dog food biscuit alone (Control; n=5), a biscuit with coumestrol diluted in dimethyl sulfoxide (COU; n=5), or a biscuit with only dimethyl sulfoxide (DMSO; n=5). a,b Different letters within sampling day indicate difference between treatments (P<0.05). *specifies statistical increase on post-treatment days 21 (P<0.0048) and 28 (0.0008). Data are presented as LSM ± standard error.
Serum E2 levels were increased by COU administration on days 21 and 28 after treatment (Figure 1B). This contrasts with findings in COU treated periovulatory stage bitches were E2 remained constant.15 Dycotomic effects of COU on other hormones and animal models have also been observed. COU given orally to ovariectomized ewes for instance, decreased amplitude of LH pulses during the breeding season, but not during anestrus.(29 Similarly, COU increased E2 secretion from granulosa cells collected from small ovarian follicles (<1 cm) of cows and heifers, but this effect was not observed when cells were obtained from larger follicles.28
An increase in serum concentrations of P4 on day 21 post-treatment (P=0.0038) was seen in DMSO group bitches (Figure 1A). Therefore, DMSO seems to exert a progestogenic effect in anestrus bitches that had not been seen in studies with periovulatory dogs, where a similar dose of DMSO was given. However, DMSO was reported to decrease P4 concentrations from day 21 to 28 post-treatment in periovulatory bitches 15. Thus, a stage-dependent effect of DMSO on P4 secretion may also be possible, although no related studies were found for other mammal species.
DMSO did not alter E2 levels during the first 28 days post-treatment (Figure 1B). This contrasts to findings in periovulatory bitches, where the same dose of DMSO increased circulating E2 on day 21 after administration15, again suggesting a stage-dependent effect. There is evidence in animals other than mammals, of DMSO-mediated effects on E2 receptors (ER). Indeed, DMSO stimulates ERα and ERβ in salmon hepatocytes.19 StAR and P450scc enzymes are also affected by DMSO in rat brain and kidneys.30
Levels of P4 and E2 remained constant and did not differ between groups from the second to the sixth post-treatment months (Table 1). This suggests that even when there is a delayed effect of COU and/or DMSO administration (first detected on day 21 post-treatment), this effect is relatively short, since no changes in hormone levels were observed beyond day 28. In particular for COU, this result could relate to an interaction between COU and the ER.32 There is no evidence of DMSO binding to ERs but it can increase ERα and ERβ in salmon hepatocytes,19 thus it may have direct or indirect actions through the E2 pathway.
Table 1 Serum levels of progesterone and estradiol in anestrus bitches from the second to the sixth month after treatment.
| Month after treatment | ||||||
| Hormone | Group | 2 | 3 | 4 | 5 | 6 |
| P4(ng/mL) | Control | 9.4 ± 4.9 | 39.7 ± 23.4 | 50.9 ± 29.2 | 24.1 ± 12.9 | 5.3 ± 5.0 |
| COU | 15.5 ± 6.5 | 6.8 ± 2.8 | 18.3 ± 11.2 | 3.3 ± 1.4 | 0.3 ± 0.1 | |
| DMSO | 25.3 ± 19.0 | 18.8 ± 10.8 | 3.4 ± 1.2 | 52.2 ± 24.2 | 3.1 ± 1.3 | |
| E2 (pg/mL) | Control | 33.8 ± 15.1 | 38.5 ± 17.3 | 45.1 ± 20.2 | 53.9 ± 24.1 | 39.1 ± 17.5 |
| COU | 14.8 ± 5.3 | 52.8 ± 15.5 | 49.5 ± 5.3 | 42.8 ± 8.1 | 34.2 ± 4.4 | |
| DMSO | 246.7 ± 196.4 | 39.5 ± 25.6 | 12.8 ± 1.5 | 72.2 ± 34.6 | 68.6 ± 34.8 | |
Serum levels of progesterone (P4), and estradiol (E2) in anestrus bitches given a single commercial dog food biscuit (Control, n=5); a single biscuit with coumestrol dissolved in dimethyl sulfoxide (COU, n=5) or a single biscuit with only dimethyl sulfoxide (DMSO, n=5). Data are presented as mean ± standard error.
Vaginal cells
A single administration of COU did not affect types (Table 2) or numbers (Figure 2) of vaginal cells in anestrus bitches; whereas number of parabasal cells was reduced and that of superficial anucleated cells increased in periovulatory animals15. Again, this difference could come down to the stage of the estrous cycle when treatment was applied. However, this may not be the case15 for other species. COU and other phytoestrogens induce a cornification of the vaginal epithelium that is independent of reproductive status or age in rodents. Similarly, observed changes in vaginal epithelium of women appeared to be more related to phytoestrogen consumption than to reproductive state.33
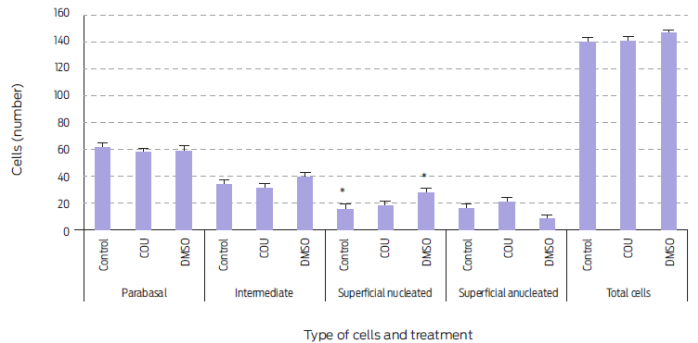
Figure 2 Vaginal cell type and number during the first 28 days post-treatment in anestrus bitches receiving a single commercial dog food biscuit (Control, n=5), a biscuit with added coumestrol dissolved in dimethyl sulfoxide (COU, n=5), or a single biscuit with only dimethyl sulfoxide (DMSO, n=5)., Means sharing an asterisk within cell type differ (P=0.056). Data are presented as mean ± standard error.
Table 2 Proportion of vaginal cell type in anestrus bitches treated with coumestrol and/or dimethyl sulfoxide.
| Day | Group | Vaginal cells | |||
| Parabasal | Intermediate | Superficial | |||
| Nucleated | Anucleat ed | ||||
| 0 | Control | 46.4 ± 5.6 | 21.6 ± 1.4 | 13.5 ± 2.1 | 18.5 ± 3.8 |
| COU | 50.1 ± 3.4 | 24.1 ± 5.6 | 8.6 ± 1.0 | 17.6 ± 6.1 | |
| DMSO | 45.4 ± 1.6 | 32.8 ± 6.5 | 18.4 ± 5.8 | 4.5 ± 1.7 | |
| 7 | Control | 41.1 ± 2.2 | 32.6 ± 1.5 | 10.1 ± 0.4 | 16.2 ± 2.5 |
| COU | 45.8 ± 1.1 | 20.3 ± 7.0 | 13.7 ± 1.7 | 20.6 ± 7.0 | |
| DMSO | 49.1 ± 3.1 | 30.9 ± 5.3 | 17.4 ± 4.6 | 5.1 ± 2 | |
| 14 | Control | 54.3 ± 5.1 | 23.7 ± 3.1 | 9.8 ± 3.5 | 12.1 ± 2.4 |
| COU | 47.0 ± 1.7 | 22.5 ± 7.7 | 13.1 ± 2.2 | 18.2 ± 9.0 | |
| DMSO | 52.8 ± 5.0 | 29.1 ± 4.8 | 16.4 ± 3.9 | 5.7 ± 2.4 | |
| 21 | Control | 49.5 ± 5.0 | 30.4 ± 6.6 | 12.3 ± 4.3 | 8.8 ± 7.9 |
| COU | 48.9 ± 1.5 | 24.6 ± 6.7 | 1 6.6 ± 2.8 | 10.9 ± 5.6 | |
| DMSO | 50.8 ± 6.8 | 23.2 ± 6.8 | 26.8 ± 7.8 | 5.6 ± 3.5 | |
| 28 | Control | 51.1 ± 4.7 | 28.1 ± 4.0 | 9.9 ± 4.1 a | 10.8 ± 1.6 |
| COU | 49.5 ± 3.7 | 20.6 ± 5.9 | 13.3 ± 4.6 a | 17.1 ± 5.2 | |
| DMSO | 45.3 ± 1.3 | 20.0 ± 6.1 | 30.4 ± 4.3 b | 6.3 ± 4.5 | |
Proportion of vaginal cell type in anestrus bitches given a single commercial dog food biscuit (Control, n=5); a biscuit with added coumestrol dissolved in dimethyl sulfoxide (COU, n=5), or single biscuit with only dimethyl sulfoxide (DMSO, n=5). a, b Different superscripts indicate difference within day (P=0.007). Data are presented as mean ± standard error.
DMSO, when administered alone, did increase the number of superficial nucleated cells at day 28 post-treatment (P=0.007; Table 2). In fact, superficial nucleated cell number was higher during the post-treatment day 28 in DMSO treated animals than in any of the other groups ((P=0.056; Figure 2). Thus, DMSO showed an estrogenic effect in vaginal cells without an observed increase in either P4 or E2 serum levels. An enhanced number of parabasal cells after DMSO administration has been reported in periovulatory stage bitches.15 Hence, DMSO may have different effects on vaginal epithelium according to the stage of the estrous cycle at which it is given.
Diestrus and anestrus lengths
Length of anestrus was not affected by treatment (Figure 3). However, diestrus was longer in the DMSO group when compared to control (P=0.060) or COU (P=0.018) treated animals. The physiological mechanism to explain this result, was not assessed in this study. However, since DMSO increased serum levels of P4 on day 21 post-treatment, a cystic-like effect could have been induced which may have prolonged diestrus.
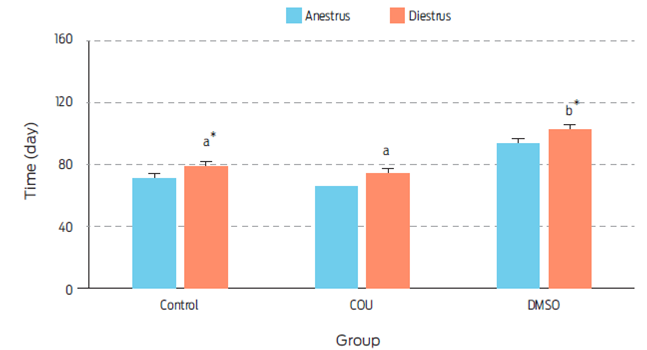
Figure 3 Length of anestrus and diestrus (days) on bitches receiving a single commercial dog food biscuit (Control, n=5), a single biscuit with added coumestrol dissolved in dimethyl sulfoxide (COU, n=5), or a single biscuit with dimethyl sulfoxide alone (DMSO, n=5). a, b Different superscripts indicate difference between treatments (P=0.018). , Means sharing an asterisk within groups differ (P=0.060). Data are presented as Least square means ± standard error.
Health and side effects
At the beginning of the study all bitches were deemed as healthy after clinical examination, and all blood parameters were within reference values. No health or behavioral alterations were observed in any of the animals during the six-month trial period. This coincides with previous work where COU and DMSO treated periovulatory-stage bitches were found healthy throughout the study, and had normal blood panels six months after treatment.15 Thus, a single oral administration of COU and/or DMSO does not appear to negatively affect health status of treated bitches. However, two of the COU-treated animals, showed abnormal mammary gland growth and galactorrhea during the third month after treatment which endured for close to 15 days (bitches identified as COU-3 and COU-5; Figure 4). Furthermore, an animal from the DMSO treatment group (bitch DMSO-4), showed galactorrhea, with no apparent mammary growth on month 6 post-treatment. In addition, a dog from the DMSO group (DMSO-5) had serum P4 levels ≥ 3.7ng/mL, which lasted for at least six months. The side effects of phytoestrogens on mammary gland have been documented before in ovariectomized macaques,35 and reports indicate that DMSO can induce differentiation of mammary cells from rats.20 This unwanted side effect should be thought about if inclusion of COU or DMSO is to be considered in reproductive control programs. The induced abnormal mammary gland development and galactorrhea were not considered as signs of disease in this study, since use of estrogenic compounds combined with P4 is frequent to induce lactations in infertile ruminants,36-38 in non-human primates39 and in human surrogate pregnancies.40 Moreover, care was taken to instruct the owners on how to check for behavioral changes typical of pseudopregnancy, such as circling, digging or nesting, but none were seen in any of the bitches presenting with mammary alterations in size and/or function (Figure 4).26 Finally, dogs that showed abnormal mammary development and galactorrhea also had low serum P4 levels, which does not agree with the relatively high concentrations of the hormone that are usually seen with pseudopregnancy.41 Thus, it would be unlikely to induce pseudopregnancy with either of the employed COU or DMSO doses. Nonetheless, even if there were no observed negative effects of COU and/or DMSO on health of bitches in this study, caution must be taken if frequency or dose of administration are increased, since there is evidence of induced health alterations with prolonged administration of COU in rodents and humans,14 and of DMSO in dogs, pigs and rodents42.
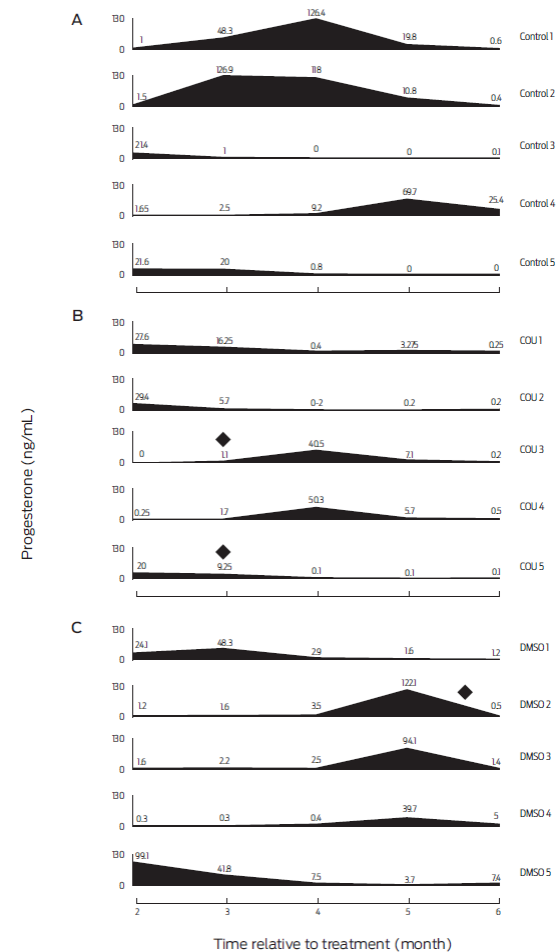
Figure 4 Serum progesterone profiles of individual bitches from months 2-6 post-treatment. A: untreated animals (Control; n=5). B: bitches receiving coumestrol diluted in dimethyl sulfoxide (COU; n=5), and C: animals receiving dimethyl sulfoxide alone (DMSO; n=5). (♦) Indicates dogs developing abnormal mammary gland growth and galactorrhea.
A shortcoming of this study was the impossibility of controlling animal diets. Dogs were given different brands of food and had diverse eating habits, which in some cases were even changed during the trial period. This made it impossible to track phytoestrogen content in given rations. However, as a reference, samples were collected from 24 different brands of commercial dog food that contained soy. Isoflavones such as genistein, daidzein, and glycitein were found, but not COU.46 We thus acknowledge that some amount of phytoestrogens may have been present in food and that this could have influenced our results. However, due to our findings regarding levels of P4 and E2, as well as on vaginal cytology patterns in control bitches, it is unlikely that the assumed presence of phytoestrogens was high enough to bias our data.
Since DMSO has been shown to have intrinsic estrogenic effects, reevaluation of studies in different mammals (such as bats, mice, and dogs) of effects previously attributed to COU (when diluted with DMSO) should be encouraged.11,16,47 Further work to investigate the mechanisms of possible effects of DMSO on reproduction are also needed.
The idea of using COU as a potential alternative to control dog overpopulation has merit. However, its potential is hindered by the impossibility to separate COU and DMSO effects in our findings, and by the unwanted side effects observed in 40% (2/5) of the bitches. As for the use of DMSO alone, our evidence shows that can act as an endocrine disruptor in bitches, extending diestrus, enhancing vaginal cell proliferation and inducing galactorrhea in 20% of the dogs. Thus, use of COU and/or DMSO in reproductive control programs does not seem viable.
Conclusions and repercussions
Our results show that COU is estrogenic and inert in vaginal cells; and that DMSO increases P4 levels, enhances vaginal cell proliferation and prolongs diestrus length. Health of bitches was not altered by treatment, but COU and/or DMSO could induce abnormal mammary growth and/or galactorrhea. The main finding of this study is the profound effect that a single oral dose of DMSO can have on reproduction of female dogs. Reevaluation of effects attributed to COU (when diluted in DMSO) in this and previous reports is encouraged.











 nueva página del texto (beta)
nueva página del texto (beta)


