Introduction
C-Phycocyanin (C-PC) is a constituent biopigment of Arthrospira maxima, a cyanobacteria native to the alkaline habitat of the Texcoco Lake in Central Mexico. It can be overproduced when these bacteria are grown under high urea and salt concentrations (yields of 220 mg C-PC g−1 biomass).1 C-PC has a protein and a non-protein component (containing three chromophores), which can be very reactive to other proteins. The protein element of C-PC has two α and a β subunits. When added as a protein supplement in an extender for semen preservation, it can react with membrane proteins of cells. The protein and the non-protein (phycocyanobilin) elements of the compound are coupled through cystein and thioether linkages.2 The non-protein component is able to capture and prevent the formation of reactive nitrogen and oxygen species (RNS & ROS) through the scavenging of hydroxyl and ( ● OH) alkoxyl (RO ● ) radicals, hydrogen peroxide (H2O2) and nitric oxide (NO ● ).2 It can thus delay, reduce or avert essential molecule impairment,3 caused by the unbalance of ROS production and an alteration in the antioxidant defense system.4,5 Oxidative stress (OS) is related to the ageing process of cells.2,6 OS can also lead to a loss of cell membrane integrity due to increased permeability, enzyme inactivation, DNA damage and cell apoptosis.7,8 For sperm in particular, these alterations are related to male infertility.9,10 Notably, during mammal sperm capacitation, which is an oxidative process, a limited amount of ROS production is needed.11,12
Pig sperm is considered a model system to study oxidative stress due to an abundant presence of unsaturated fatty acids in the plasmatic membrane that can render it highly vulnerable.13,14 Even if boar semen can be cryopreserved,13 the best results for transient storage are attained at 15−17 °C.15,16 It seems that when boar sperm is stored at 16 °C it has a lower susceptibility to DNA damage, as alterations in chromatin packaging occur at lower temperatures (5 °C).17 However, storing sperm at 15-17 °C could be difficult under field conditions, since regular available refrigerators keep a fairly constant 4 °C temperature.
Sperm storage is essential for reproductive biotechnologies such as artificial insemination. Extenders used for semen processing need to protect sperm, inhibit bacterial growth, provide an energy source, and ensure proper pH and osmotic pressure. A wide range of commercial extenders have been developed for optimal preservation of boar semen at 15 a 17 °C. These extenders are intended for short (3 to 4 days), medium (5 to 6 days), long (7 to 8 days), or extra-long term (more than 8 days) storage. However, information regarding extender effects on fertility of refrigerated boar sperm is scarce.15
Kortowo-3 is a short-term extender which has been used for boar semen storage at 5 °C.18 When protein is added to the extender, spermatic parameters are enhanced.17 Egg yolk is frequently used as a source of protein for ram and boar semen extenders.19,20 Furthermore, both egg yolk and milk are commonly used as membrane cryoprotectors in refrigerated media for long-term semen preservation. These components can be used either alone or in combination with other proteins.19
The aim of this study was to evaluate the effect of adding the C-PC compound to the Kortowo-3 extender on refrigerated (4 °C) boar semen parameters.
Materials and methods
Chemicals
Used chemicals were of the highest available purity. Salts were purchased from Sigma-Aldrich, Meyer and JT Baker; Eosin was acquired from Merck; and nigrosine, acridine orange, gentamicin sulfate and 2’, 7’-dichloridefluoresceine diacetate from Sigma-Aldrich.
Duragen solution (Magapor-Spain), an extender typically used for extra long-term storage, was used for semen transport.
C-PC source
C-PC was obtained from the A. maxima LJGR1 strain from the collection of the Ecology and Taxonomy of Continental Algae Laboratory at the Universidad Nacional Autónoma de México (UNAM). Bacteria were grown under stress conditions to incite overproduction of C-PC, which was subsequently extracted by sonication at 45 °C, purified (0.7) and freeze-dried until used.1
Boar sperm
Five one-year old boars (Pietrain) were used for the study. Fourteen semen samples were collected, filtered, diluted in Duragen solution, and transported at 17 °C (protected from light), to the laboratory. Inclusion parameters for ejaculates were set at more than 90% viability, 70% progressive motility, less than 10% abnormalities and over 25 million sperm cells mL−1.21,22 Twelve samples attained adequate quality to be included in the experiments.
To assess sperm motility, 5 µL of the sample (around 200 cells) were observed under a bright field microscope at 40X to identify: progressive motility, non-progressive motility and immobile cells.
To calculate spermatozoa concentration an improved Neubauer hemocytometer was used.22
Viability and sperm abnormalities were examined using an eosine-nigrosine stain. A slide was prepared by mixing 5 µL of the semen sample with 5 µL of a 10% eosine-nigrosine solution on a slide, left to dry for 30 seconds and observed using a bright field microscope. Identification of unstained live cells and of purple-stained dead cells was done with at 40X magnification. Percentage of cells presenting morphological abnormalities or exhibiting excess residual cytoplasm was determined with a 100X objective.22
Sperm storage with C-PC
Semen samples were centrifuged upon arrival to the laboratory at 600 g for 10 minutes (in a Zeigen CH90−1A centrifuge) to remove the Duragen solution. Sediment was then diluted to a 30x106 spermatozoa mL−1 cell count, using the Kortowo-3 extender (fructose 69.3 mM, sodium citrate 64.6 mM, Na2-EDTA 8 mM, potassium acetate 14.2 mM and gentamicine 0.25 g L−1 at pH 6.8).18 Diluted samples were divided, and five different C-PC doses were added to the solution (0, 34.5, 69, 138 and 207 µM). Samples were left for 2 hours at room temperature and subsequently refrigerated at 4 °C for 24, 48 and 72 hours. At each time point, semen samples were assessed for sperm motility, viability, DNA damage and ROS production.
Assessment of DNA damage
Flow cytometry assessment of DNA damage was performed with a 488 nm argon blue laser with acridine orange to show fluorescence (FACScan, Becton-Dickinson). A green color emision (530 nm) revealed undamaged double strand DNA, whereas a red/orange emission (620 nm) indicated damage on a single DNA strand.23-25
Quantification of reactive oxygen species (ROS)
Intracellular ROS production (H2O2, HO ● and ONOO ● ) in sperm was quantified by flow cytometry (480 nm/520 nm; FACScan, Becton-Dickinson, San Jose, CA, USA), using 2’,7’ dichlorofluoreseine diacetate (DCFH-DA), a cell-permeable probe that is highly sensitive to cellular oxidation and fluoresces when oxidized. Results are expressed as mean fluorescence intensity (MFI) of live cells exhibiting a fluorescent response.26 The Flowing software was used to analyze data, which were normalized and expressed as fluorescence index (FI) = (mean fluorescence intensity * % input events).
Results and discussion
Sperm viability decreases after being stored for 24 hours, falling from 90% to close to 10% by 72 hours. Sperm viability at 72h was higher with the 138 µg mL−1 C-PC dose (16 ± 6%). Considering that at least a 20% viability is required for sperm use in assisted reproduction techniques boar refrigerated sperm would not be fit for use after 72 h of storage, even with addition of C-PC (Figure 1).
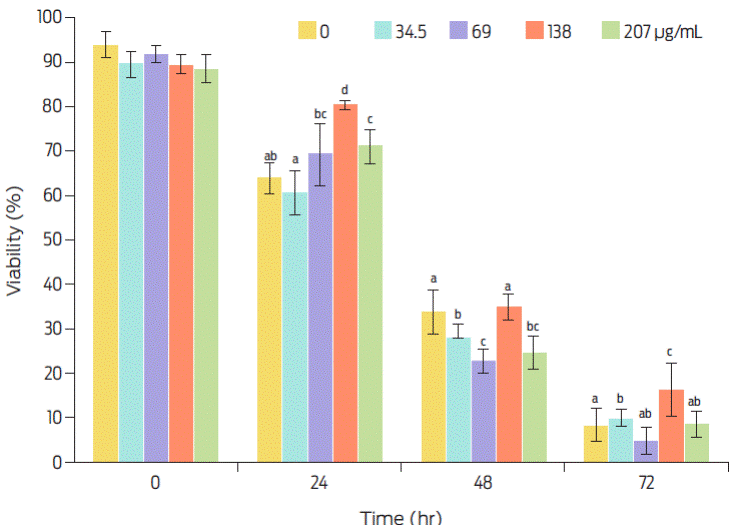
Figure 1 Effect of C-PC dose and storage time (at 4 °C), on sperm viability. Values are expressed as means ± SEM. Different superscripts indicate statistical difference as analyzed by ANOVA and the Duncan multiple comparisons test (P≤0.05).
Progressive motility (PMot), which is affected by intracellular ROS production, was close to 90% in viable sperm before storage (Figure 2), whereas DNA damage was present in near 40% of all spermatozoa, independently of storage time (Figure 3). Therefore, the proportion of motile sperm that also has undamaged DNA is over 40% before storage and decreases thereafter with increasing storage time (Figure 4). It seems likely that the protein element of C-PC as sole added protein source to the extender is insufficient for protecting the sperm cell membrane. Conversely, the antioxidant element of C-PC seems to have had a positive effect on controlling intracellular ROS production, as seen by the mean fluorescence intensity (MIF) of viable cells (Figure 5).

Figure 2 Effect of C-PC dose and storage time (at 4 °C) on progressive motility of sperm. Values are expressed as means ± SEM. Different superscripts indicate statistical difference as analyzed by ANOVA and the Duncan multiple comparisons test (P≤0.05).
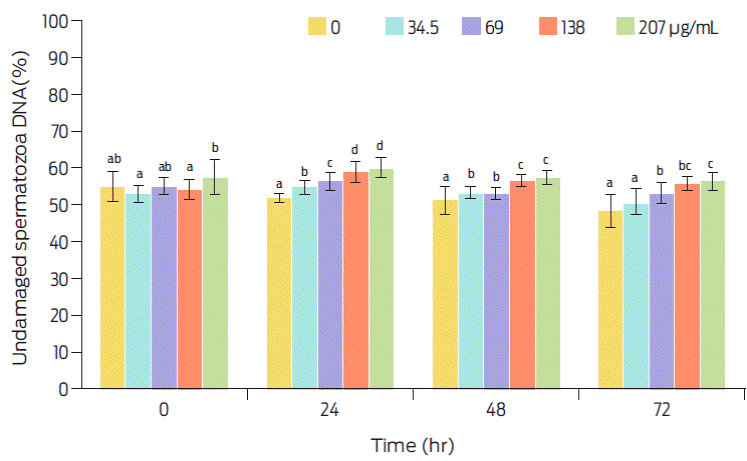
Figure 3 Effect of C-PC dose and storage time (at 4 °C), on sperm DNA damage. Values are expressed as means ± SEM. Different superscripts indicate statistical difference as analyzed by ANOVA and the Duncan multiple comparisons test (P≤0.05).
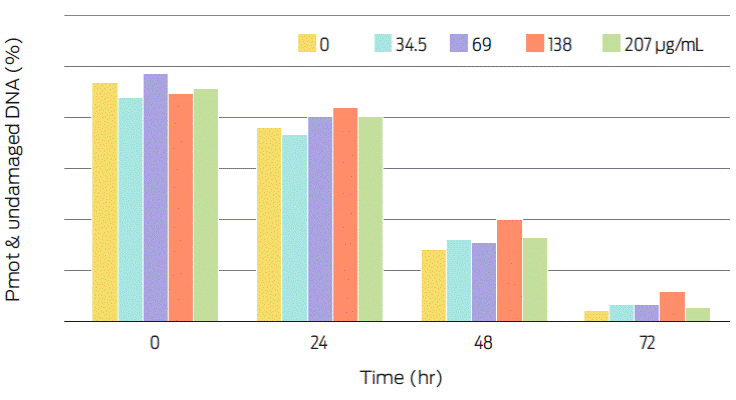
Figure 4 Percentage of PMot sperm with undamaged DNA. Treatments include different C-PC doses and storage times (at 4 °C). .PMot=Progressive motility.
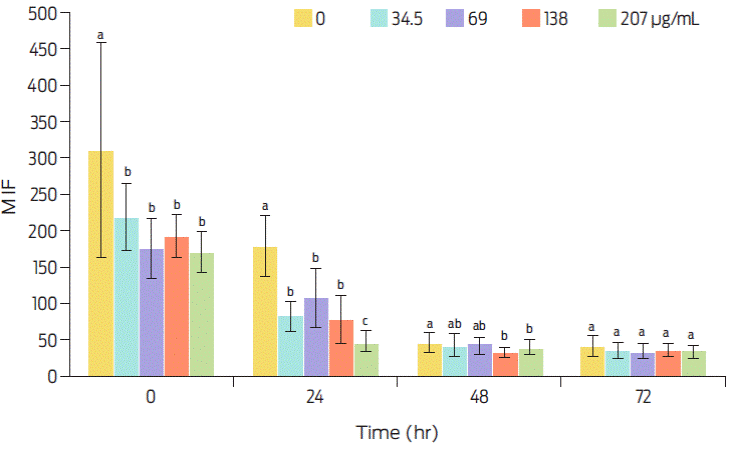
Figure 5 Effect of C-PC dose and storage time (at 4 °C), on intracellular ROS production, as seen by percentage of mean fluorescence intensity (MFI) of viable cells. Values are expressed as means ±SEM. Different superscripts indicate statistical difference as analyzed by ANOVA and the Duncan multiple comparisons test (P≤0.05).
Sperm storage at temperatures lower than 5 °C can induce cold stress on boar spermatozoa,16 which can then cause irreversible permeability of cells through loss of integrity of the plasmatic membrane. This may lead to a distorted cell performance and eventually to cell death. Proteins that are diluted in processing media have been able to protect spermatozoa from cold stress and plasmatic membrane destabilization,18 and are thus added to extenders used for handling semen.17,19,20
The Kortowo-3 extender has been used for refrigerated semen studies17,18 with reported sperm progressive motility percentages similar to the ones obtained in this work. However, if the extender is supplemented with chicken lipoprotein and ostrich egg yolk, PMot is higher (70%) at 48 hours, than that of semen with an extender that is devoid of added proteins.17 Our results show a 35% of PMot with 138 µg of added C-PC mL−1 at 48 hours, and a 28% PMot when no C-PC was added to the extender (Figure 2). Therefore, the C-PC apoprotein does not seem to work as well as other proteins (egg yolk or lipoproteins) to increase this spermatic parameter. Boar DNA has a higher resistance to the freezing-thawing process than other mammalian spermatozoa. This resistance seems to be related to the amount of cysteine residues in protamine 1 which forms disulphide bonds that stabilize chromatin. Nonetheless cysteine residues have also been negatively related to sperm susceptibility to oxidative stress. In fact, bioactive antioxidant compounds of mate tea (such as flavonoids) can protect boar sperm DNA.27 These seemingly contradictory data can help explain why percentage of sperm cells without DNA damage appears to increase with some of the added C-PC doses, as well as over time (Figure 3). Extender should protect sperm without interfering with fertility or prolificacy rates.15 Progressive motility on sperm with undamaged DNA after 48 hours of storage was still close to 20% (Figure 4).
Conclusion
The protein element of C-PC does not have a positive effect on sperm viability when added to the Kortowo-3 short-term extender. However, the antioxidant portion of the C-PC compound appears to decrease ROS production in cells, and could be therefore used as antioxidant in short and long-term storage extenders.











 nova página do texto(beta)
nova página do texto(beta)


