Introduction
In vitro embryo production by somatic cell nuclear transfer (SCNT) is a reproductive biotechnology with great potential for the propagation of genetically valuable individuals, farm animal production and the preservation of endangered wild species.1 This technology can also be a useful tool for basic research into knowledge on embryo development and nuclear reprogramming processes. SCNT can be defined as the process that makes it possible to obtain one or more genetically identical individuals from a single nucleus or somatic cell.2 Diverse domestic animals, such as sheep, monkeys, cattle, mice, goats, pigs, cats, rabbits, rats, mules, horses, dogs and ferrets,3 and wild species, such as gaurs,4 European mouflon,5 buffalo,6 gray wolves,7 and camels, have been cloned.8
Traditionally, in SCNT, micromanipulators are used to enucleate oocytes and introduce genetic material from the individuals to be cloned. A variant of the technique is handmade cloning (HMC), which does not use micromanipulators, making it an accessible and less complex technique.9 Using this technique, porcine,10 bovine11 and ovine12 clones have been obtained. For both cloning techniques, efficiency is still low; depending on the species, only 1-5 % of the transferred embryos develop into viable offspring, and cloned animals have shown developmental problems in other cases.13 These problems could be due to the complexity of the technique, which involves several factors that can affect embryo development.14 In this regard, Camargo et al.15 stated that oocyte in vitro maturation is the main factor for obtaining optimal in vitro embryo development.
An important factor that can affect in vitro maturation (IVM) is oxidative stress, which causes an imbalance between oxidant and antioxidant levels inside the cell.16 It has been reported that oocyte maturation is strongly influenced by glutathione and reactive oxygen species (ROS) levels.17 ROS are oxygen metabolism derivatives formed by the breakdown, excitation or reduction of molecular oxygen.18 ROS overproduction during IVM and early embryo development can produce lipid peroxidation in the cell membrane, DNA fragmentation, and alterations in RNA transcription and protein synthesis.16 These events can cause oocyte and embryo damage and may even cause cell death. Therefore, it is necessary to establish an IVM system that is capable of reducing intracellular ROS levels as well as oxidative damage that reduce oocyte quality, contributing to improved embryo development. The use of several antioxidants, such as b-mercaptoethanol, cysteine, cystine and L-carnitine, has been proposed,17,19 as well as ascorbic acid and a-tocopherol (vitamin C and vitamin E, respectively).20 Recently, it has been reported that resveratrol (trans-resveratrol) used during IVM increases embryo development in porcine, bovine and caprine species.21-23
Resveratrol (3,4’,5-trihydroxystilbene) is a natural polyphenol present in plants such as polygonum (Polygonum cuspidatum) and the common grape vine (Vitis vinifera), and it has biological activity to protect plants from the attack of pathogens, fungi and bacteria. Resveratrol has antioxidant, anti-inflammatory, cardioprotective and anti-cancer effects,24 and it regulates gene expression related to apoptosis by activating sirtuin 1 (SIRT1) and increasing mitochondrial function and the ATP content in oocytes.25
The present study evaluated the effects of different resveratrol concentrations on IVM and the level of ROS in Ovis aries oocytes, as well as on handmade cloned embryo quality and development rate.
Materials and methods
Unless otherwise specified, all chemicals and reagents used in this study were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). All techniques were performed under sterile conditions in a laminar flow hood. Incubation was always at 38 °C and 5 % CO2 in a 95 % humidified atmosphere. The present experiments complied with relevant institutional animal welfare guidelines, such as the Guidelines for the Ethical Conduct of Research, Teaching and Diffusion of the Biological and Health Sciences Division from the Universidad Autónoma Metropolitana and were approved by the Divisional Council of Biological and Health Sciences Division in Session 8.10 on May 18th, 2010 in accordance with the national animal welfare guidelines (NOM-062-ZOO-1999; NOM-033-ZOO-1995) and the policies and ethics committee approval (Academic Commission of Ethics of the Biological and Health Sciences Division at Universidad Autónoma Metropolitana).
Oocyte collection and in vitro maturation
The ovaries were obtained from adult Ovis aries Criollo sheep from a slaughterhouse in Nezahualcoyotl, Estado de Mexico and transported to the laboratory at 28-35 °C in saline solution [0.9 % NaCl and 1 % antibiotics (10, 000 UI/mL penicillin, 10 mg/mL streptomycin sulfate and 25 µg/mL amphotericin) (Antibac-Antifun 100x, In Vitro, SA)] within two hours. The ovaries were washed three times in isothermal saline solution. The cumulus oocyte complexes (COCs) were punctured within a 2-6 mm diameter of the ovary follicles and aspirated using a 20 gauge hypodermic needle attached to a 10 mL disposable syringe containing 1 mL of HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffered TCM-199 (In Vitro, SA) and 100 UI/mL heparin.26 Once the follicular fluid was obtained, the COCs with a homogeneous cytoplasm and at least three layers of cumulus cells were selected for IVM, based on the Kakkassery et al.27 criteria. The COCs were washed three times in maturation medium [TCM-199 containing 10 % fetal bovine serum (FBS) (Microlab), 1 % epidermal growth factor (EGF), 5 µg/mL follicle stimulating hormone (FSH) (Folltropin-V, Bioniche), 5 UI/mL chorionic gonadotrophin (CG) (Loeffler), and 0.6 % antibiotics]. Groups of 25 to 30 COCs were cultured in four-well dishes with 500 µL of maturation medium under 300 µL of mineral oil and incubated for 24 hours.28 During IVM, the COCs were treated with different concentrations of resveratrol (0, 0.5, 2 and 5 µM). The resveratrol was dissolved in dimethyl sulfoxide (DMSO) as a 500 µM stock solution and stored at −20 °C before being added to the maturation medium.23
Assessment of nuclear maturation
After IVM, the COCs with at least four cumulus cell layers were selected and denuded by incubation in HEPES-buffered TCM-199 containing 0.5 mg/mL hyaluronidase for a minute and vortexed at maximal speed for three minutes to remove the cumulus cells. The nuclear status of the oocytes was examined by use of a stereoscopic microscope to identify the presence or absence of the first polar body. The oocytes with first polar body extrusion were considered mature, and those without polar body extrusion were considered immature.29
Measurement of intracellular ROS levels
The matured oocytes were sampled to measure intracellular ROS levels by a 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescence assay. An average of six oocytes from each treatment group were incubated in darkness in TCM-199 containing 10 µM of H2DCFDA for 30 minutes. Following incubation, the oocytes were washed three times in 100 µL of HEPES-buffered TCM-199 and fixed onto slides. The fluorescence was observed under an epifluorescence microscope equipped with a 460 nm filter. The fluorescent images were saved as JPG files, and the fluorescent intensities of the oocytes were analyzed by ImageJ software (Version 1.41; National Institute of Health, Bethesda, MD, USA).
Handmade cloning
Preparation of the recipient cytoplast
The cytoplasts were prepared as described by Vajta et al.30 Mature oocytes with expanded cumulus cells were transferred into a microcentrifuge tube containing 0.5 mg/mL hyaluronidase in HEPES-buffered TCM-199, incubated for a minute and vortexed at maximal speed for three minutes. Then, the oocytes were transferred to a culture dish containing T2 (T denotes HEPES-buffered TCM-199, and the number denotes the percentage of FBS), incubated with 10 µg/mL demecolcine in TCM-199 for 40 min and washed twice in T2. The denuded oocytes were placed into 30 µL pronase (2 mg/mL in T10) for 5-10 min to digest the zona pellucida and then transferred into 20 µL T20 to stop the activity of the pronase enzyme. The zona-free oocytes were examined by use of a stereoscopic microscope, and those with a homogeneous cytoplasm and first polar body extrusion were selected. The selected zona-free oocytes were transferred into 20 µL T10 and enucleated by manual bisection using an ultrasharp microblade (Ultra-Sharp Splitting Blade, Bioniche) to remove the polar body and nuclear material. The enucleated oocytes were transferred to T20 and incubated until the enucleation of all oocytes was finished.
Preparation of the donor cells (karyoplast)
A 1 cm2 ear skin biopsy was taken aseptically from a five-month-post-mortem female fetus of a Criollo sheep (Ovis aries) from a slaughterhouse in Nezahualcoyotl, Estado de Mexico. The tissue was placed in a 1.5 mL conical tube containing a milliliter of Dulbecco’s phosphate buffer saline (DPBS) with 2 % antibiotics and transported to the laboratory on ice within two hours. The biopsy was then disinfected with 1 % of chlorine for three minutes and 70 % alcohol for three seconds and washed three times in DPBS for a minute each. Then, the tissue was mechanically fragmented into small pieces and chemically digested with collagenase type I (2 mg/mL, Gibco) and type II (2 mg/mL, Gibco) in an oscillator inside a drying oven at 37 °C for an hour. Five milliliters of supplemented Dulbecco’s Eagle modified medium (DMEM) (10 % FBS and 2 % antibiotics) was added to stop the action of both collagenases, and the sample was centrifuged at 500/g for ten minutes to produce a cell pellet. The pellet was resuspended in three milliliters of supplemented DMEM in a culture dish and incubated for seven days until the cells reached confluence. When the cells (fibroblasts) achieved 100 % confluence, the cells were passaged. The culture medium was removed from the culture dish, and the culture dish was then washed three times with 500 µL of DPBS. The cells were incubated with 700 µL of trypsin-versene (0.05/0.05 %) (In Vitro, SA) for five minutes. Then, 700 µL of supplemented DMEM was added, and the cells were centrifuged at 150/g for four minutes to produce a cell pellet. The pellet was recovered and divided into two halves. One half was resuspended in three milliliters of supplemented DMEM and incubated for seven days until the cells reached confluence, and then the cells were passaged. The other half was resuspended in 500 µL of DMEM and kept at room temperature until cell fusion.28 The cells were then ready for use as nucleus donors or karyoplasts.
Pairing and electrofusion of karyoplasts and cytoplasts
Two cytoplasts were immersed in 15 µL phytohemagglutinin (5 mg/mL in HEPES-buffered TCM-199) for 5-10 seconds and then transferred to 15 µL T2 containing approximately 30 karyoplasts previously placed into the solution. The two cytoplasts were attached to a karyoplast forming a triplet, which was transferred to 15 µL fusion medium (5.46 g D-mannitol and 0.1 g polyvinyl alcohol in 98 mL H2O) and placed between the two electrodes in the fusion chamber (BTX Microslide. Model 450; BTX, San Diego, CA) covered with 200 µL fusion medium. Then, the triplet was aligned between both electrodes of the chamber with an AC pulse (4 V) using the BLS electrofusion instrument (CF-150/B. Budapest, Hungary), and a single DC pulse (105 V) was applied for 9 µs for fusion. The fused triplets that acquired a spherical form were considered cloned embryos and were transferred to SOF medium (In Vitro, SA) containing 5 % FBS and incubated for two hours for reprogramming.30
Activation and in vitro culture of cloned embryos
The chemical activation of the cloned embryos was achieved by incubation in 5 µM Ca2+ ionophore in T2 for five minutes, followed by washing three times in T20. Then, the embryos were individually incubated in 2 µL droplets of SOF containing 5 % FBS and 2 mM 6-dimethylaminopurine (6-DMAP) covered with mineral oil for four hours and washed three times with SOF containing 5 % FBS. Finally, the activated embryos were cultured using the well of the wells (WOW) system, as described by Vajta et al.30 The microwells were prepared with a v-shaped needle in the bottom of each well of a four-well dish and contained 500 µL SOF with 15 % FBS and covered with 300 µL mineral oil. The embryos were carefully loaded individually into the microwells and incubated for seven days.
Assessment of embryo development and morphological embryo quality
The rates of embryo development were evaluated during in vitro culture (IVC). At 48, 120 and 168 h of IVC, the percent development of each stage was determined. The assessment of morphological embryo quality was examined at 120 and 168 h of IVC. The embryos were classified into embryo qualities of 1, 2, 3 and 4 (excellent, good, regular and bad or not transferrable, respectively), as described by Stringfellow and Seidel.31
Experimental design
In experiment 1, the effect of different concentrations (0, 0.5, 2 and 5 µM) of resveratrol on the IVM of ovine oocytes was determined. In experiment 2, the effect of different resveratrol concentrations during IVM on intracellular ROS levels was evaluated. In experiments 3 and 4, the effect of resveratrol treatment during IVM on the subsequent early embryonic development and morphological embryo quality of handmade cloned embryos was examined.
Statistical analysis
All statistical analyses were performed using NCSS 2007 (NCSS, LLC. Kaysville, Utah, USA). The percentage data for maturation rate, cleavage and embryonic yield were compared by one-way ANOVA followed by Duncan’s multiple range test. The data are presented as the mean ± standard deviation. Differences were considered significant at p < 0.05. The experiments were replicated at least five times.
Results and discussion
Effect of different concentrations of resveratrol on ovine oocyte IVM
The effect of different concentrations (0, 0.5, 2 and 5 µM) of resveratrol on the maturation rate of ovine oocytes was assessed; the results are presented in Table 1. No significant difference was observed between the 0.5 and 2 µM resveratrol groups (81.3 and 72 %, respectively) compared with the control (74.2 %). However, the maturation rate of the oocytes treated with 5 µM resveratrol was decreased (56 %) compared to that of the control (F(3,18) = 13.939, p = 6.085).
Table 1 Effect of different resveratrol concentrations during IVM
| Resveratrol (μM) | Oocytes (n) | % in vitro maturation (n) |
|---|---|---|
| 0 (Control) | 274 | 74.2 ± 9.5 (202)a |
| 0.5 | 261 | 81.3 ± 6.7 (215)a |
| 2 | 193 | 72.0 ± 5.9 (140)a |
| 5 | 184 | 56.0 ± 5.2 (103)b |
Values with different superscript letters within a column differ significantly (p < 0.05). Replicated eight times.
Our results revealed that resveratrol treatment during IVM resulted in concentration-dependent effects. Although the results were not significant, the treatment with 0.5 µM resveratrol was considered optimal because it tended to increase the IVM rate. In contrast, the 5 µM resveratrol treatment decreased the IVM rate significantly compared to other concentrations. Similar to these results, Mukherjee et al. 23 found a beneficial effect with 0.25 and 0.5 µM resveratrol and a detrimental effect with 5 µM on goat oocyte IVM. However, Kwak et al.21 when working with porcine oocytes, reported that resveratrol treatment during IVM had a concentration-dependent effect; 2 µM resveratrol increased the percentage of mature oocytes; however, with the highest concentration (10 µM), the percentage significantly decreased. We suggest that 5 µM resveratrol during IVM decreases the nuclear maturation rate due to an imbalance between the levels of oxidants and intracellular antioxidants. Furthermore, the decrease in oocyte nuclear maturation may be related to the ability of resveratrol to inhibit phosphodiesterase activity, which results in an increase in the concentration of cAMP,32 and high levels of cAMP inhibit the progression of meiosis, while low levels activate it.33 Considering that, resveratrol affects other functions such as cytoplasmic maturation and the regulation of apoptotic gene expression (Bax, Bak, and Caspase-3) through an optimal oxidation-reduction balance, which could contribute to optimal oocyte maturation and better embryonic and fetal development.21,23
Effect of resveratrol on intracellular ROS levels during IVM
The intracellular ROS level in in vitro matured oocytes treated with different resveratrol concentrations is shown in Figure 1. The highest ROS levels were found at 5 µM (95.5 %), while they were lower at 0.5 and 2 µM (88.3 and 84.4, respectively) compared with the levels found in the control (0 µM). However, these differences were not significant (F(3,20) = 0.53, p = 0.6625) (Figure 2).
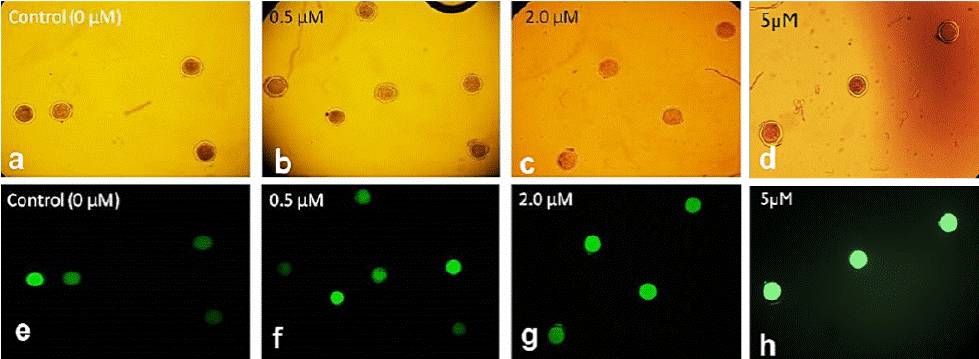
Figure 1 Epifluorescence microscope photographs of ovine oocytes treated with different resveratrol concentrations and stained with 2’,7’-dichlorodihydrofluorescein (H2DCFDA) (10x). In bright field (a-d) and with 460 nm filter (e-h).
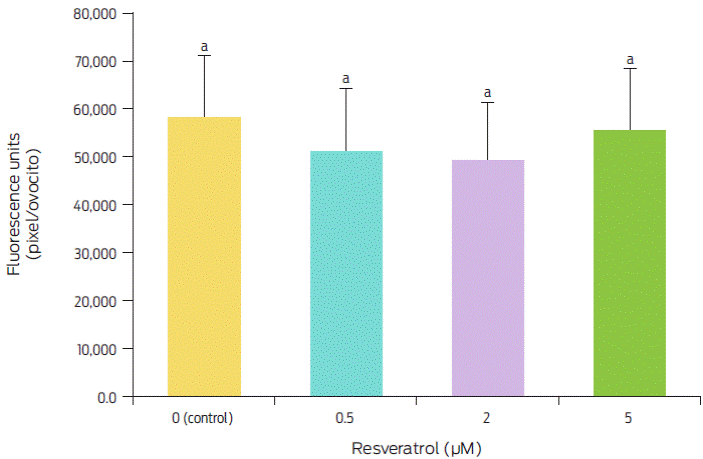
Figure 2 Effect of resveratrol treatment during IVM on the intracellular ROS levels in ovine oocytes. Bars with different superscript letters are significantly different (p < 0.05). Replicated five times.
These results differ from those reported by Mukherjee et al.,23 who evaluated ROS levels in matured goat oocytes; these authors found the highest levels with 0 and 5 µM and the lowest with 0.25 and 0.5 µM resveratrol, with a significant difference of 30 % between them. Similarly, in porcine oocytes, Kwak et al.21 obtained the highest levels of ROS with 0 and 10 µM resveratrol and the lowest levels of ROS with 0.2 and 2 µM resveratrol, with decreases of 30 % and 60 %, respectively. These authors concluded that resveratrol decreases ROS levels in matured oocytes because, at optimum concentrations, it increases intracellular GSH levels, which are strongly associated with cytoplasmic maturation and may play roles in nuclear maturation. The results obtained in this study show that, although resveratrol also decreased ROS levels, these levels were reduced by only 11.7 % to 15.6 %, which differs from the previous results of 30 %21 and 30 % to 60 %.23 These results show that resveratrol application during IVM has different effects among species, which is most likely caused by the different sensitivity of each species to this antioxidant. It is known that resveratrol enhances SIRT1 expression in oocytes and increases the expression level of phospho-5’-adenosine monophosphate-activated protein kinase in oocytes.25 However, in our study, resveratrol showed no benefits on IVM in oocytes; however, its beneficial effect was observed at the morula stage.
Effect of resveratrol treatment during IVM on the in vitro development of cloned embryos
According to developmental stage, the cloned embryos were classified into four groups: cleaved embryos, 8-16 blastomere embryos, morulae and compact morulae (Figure 3). As shown in Table 2, no differences (F(3,12) = 0.63, p = 0.608) in cleaved embryo rates were observed among the groups treated with resveratrol at 0, 0.5, 2 and 5 µM (81.2, 84.4, 80.1 and 77.2 %, respectively). Regarding 8-16 blastomere embryo rates, no difference (F(3,12) = 0.84, p = 0.049) was observed among the groups (65.6, 70.2, 64.3 and 63.6 %, respectively). However, the rate of morulae in 5 µM resveratrol (6.8 %) decreased compared to that in the control as well as 0.5 and 2 µM resveratrol (32.8, 35.1 and 28.7 %, respectively) (F(3,12) = 21.19, p = 4.36). With regard to compact morulae rates, the group treated with 0.5 µM resveratrol (10.7 %) was higher (F(3,12) = 10.14, p = 0.001) than the control group (6.2 %), while, for the groups treated with 2 and 5 µM resveratrol, there were no compact morulae.

Figure 3 Different developmental stages of the HMC sheep embryos cultured in the WOW system. Cleaved embryos (a-d), 8-16 blastomere embryos (e-h), morulae (i-l) and compact morulae (m-p) (200X).
Table 2 Effect of resveratrol treatment during IVM on handmade cloned embryo development
| Resveratrol (μM) | Embryos cultured (n) | Embryos cultured (n) | % 8-16 blastomere embryos (n) | % Morulae (n) | % Compact morulae (n) |
|---|---|---|---|---|---|
| 0 (Control) | 64 | 81.2 ± 5.1 (52)a | 65.6 ± 3.6 (42)a | 32.8 ± 7.8 (21)a | 6.2 ± 0 (4)a |
| 0.5 | 57 | 84.4 ± 9.9 (48)a | 70.2 ± 6.2 (40)a | 35.1 ± 5.9 (20)a | 10.7 ± 4.1 (6)b |
| 2 | 45 | 80.1 ± 8.1 (36)a | 64.3 ± 7.5 (29)a | 28.7 ± 3 (13)a | 0 ± 0 (0)c |
| 5 | 44 | 77.2 ± 5.2 (34)a | 63.6 ± 7.4 (28)a | 6.8 ± 4.5 (3)b | 0 ± 0 (0)c |
Values with different superscript letters within a column differ significantly (p < 0.05). The data represent as the mean ± standard deviation. Replicated five times.
During the IVC of the cloned embryos, there were no significant differences in the cleaved embryo rate among resveratrol concentrations; however, 5 µM resveratrol tended to reduce the embryonic yield at the morula stage, and this reduction was more notable at the compact morulae stage. This result shows that treatment with 5 µM resveratrol during the IVM of ovine oocytes has a deleterious long-term impact that manifests during early embryonic development. In contrast, the positive effect of the 0.5 µM resveratrol treatment during IVM was maintained during embryonic development, generating greater morulae compaction. This finding may be related to the properties of E-cadherin, which is a principal calcium-dependent molecular component of adherent junctions that is associated with the compaction of morulae and blastocyst formation.34 E-cadherin plays a key role in the differentiation of the trophectoderm and supports further embryonic development,35 and it is known that resveratrol increases E-cadherin expression in the cell by promoting its transcriptional activity.36 Similar to the results of this study, Mukherjee et al.23 reported that a 0.5 µM resveratrol concentration favors embryonic development, whereas 5 µM reduces it. In contrast, Wang et al.22 reported that a 1 µM resveratrol treatment favors bovine embryonic development, which differs from the results obtained in this work in sheep and those reported by Mukherjee et al.23 in goats. It is clear that the optimal concentration of resveratrol for each species is different, which is possibly due to the sensitivity of each species to this antioxidant. However, it is evident that there is greater similarity between the results obtained in the present work in sheep and those obtained in goats. These similarities are probably because these two species (Ovis aries and Capra hircus) are phylogenetically close since they both belong to the subfamily Caprinae and since there are many anatomical and morphological similarities between them.37
Effect of resveratrol treatment during IVM on the morphological quality of cloned embryos
After 120 h of IVC, the morphological quality of the cloned embryos showed no differences among groups. However, after 168 h of IVC, as shown in Table 3, the morulae treated with 0.5 µM resveratrol during IVM showed an increase in the percentage (30.1 %) of excellent quality embryos and a significant decrease in the percentage of poor quality embryos (4.7 %) compared to the control group (23.8 % and 23.8 %, respectively) (F(3,7) = 24.59, p < 0.0001). In contrast, with 5 µM resveratrol, the percentage of excellent quality embryos decreased (F(3,11) = 25.88, p < 0.0001).
Table 3 Effect of resveratrol treatment during IVM on the morphological quality of cloned morulae
| Resveratrol (μM) | Evaluated embryos | % Embryo quality (n) | |||
|---|---|---|---|---|---|
| 1 (Excellent) |
2 (Good) |
3 (Regular) |
4 (Bad) |
||
| 0 (Control) | 21 | 23.8 (5)a | 28.5 (6)a | 23.8 (5)a | 23.8 (5)a |
| 0.5 | 20 | 30.1 (6)a | 39.6 (8)a | 19.8 (4)a | 4.7 (2)b |
| 2 | 13 | 23.3 (3)a | 30.0 (4)a | 19.8 (3)a | 23.3 (3)a |
| 5 | 3 | 0.0 (0)b | 33.3 (1)a | 33.3 (1)a | 33.3 (1)a |
Values with different superscript letters within a column differ significantly (p < 0.05). Replicated five times.
The same positive effect detected during embryonic development was observed in the morphological quality of cloned embryos, where the 0.5 µM resveratrol treatment increased the percentage of excellent quality embryos and reduced those of poor quality. Based on the results of this work, it is clear that the effect of resveratrol increases as embryonic development continues. The deleterious effect of 5 µM resveratrol increased at the morula stage because cellular organization requires optimal functioning of embryonic gene expression at that time.38 Furthermore, the beneficial effect of 0.5 µM resveratrol during sheep oocyte IVM gradually increased, reaching a maximum at the compact morulae stage, possibly because the antioxidant effect of resveratrol improves oocyte nuclear and cytoplasmic maturation at low concentrations, maintaining the oxidative balance within the cell.23 The positive long-term impact of resveratrol may be because, at low concentrations of resveratrol during IVM, the expression of pro-apoptotic genes in the oocytes decreases.21 Resveratrol probably promotes cytoplasmic maturation, which is critical for achieving optimal embryonic development.21 Other authors reported that the beneficial effect of resveratrol is due not only to its antioxidant activity but also to its ability to regulate SIRT1 expression.22 SIRT1 is involved in the regulation of mitochondrial biogenesis, including the generation of ATP and the regulation of AMPK, which increases b-oxidation and, consequently, the consumption of fatty acids, thus improving embryo development.25 However, further studies could confirm these observations.
Conclusions
The effects of resveratrol on oocyte IVM and ROS concentrations are controversial. In this work, during sheep oocyte IVM, resveratrol did not significantly improve the nuclear maturation rate or intracellular ROS levels. However, the results suggest that 0.5 µM resveratrol during IVM improves oocyte quality and promotes morulae compaction of Ovis aries handmade cloned embryos during early development. This finding may be because resveratrol affects the expression of calcium-dependent cell adhesion molecules, such as E-cadherin, that are required for morulae compaction.











 nova página do texto(beta)
nova página do texto(beta)


