Introduction
As more effective drugs become available and fewer new breakthrough drugs emerge, clinical investigation objectives change. Clinical investigations often seek non-inferiority or equivalency effects of new drugs, rather than superiority, compared with existing effective standard drugs in active controlled trials, and the known efficacy of the standard treatment is then transformed to the new treatment. Since the interest is primarily one-sided, such trials are named “non-inferiority” trials to show that the new treatment is not inferior to the standard treatment by more than a small, predefined margin.1
Demand for bovine meat has steadily increased in recent decades,2 and pharmacological intervention with zilpaterol hydrochloride (ZH) has been used to increase production. This drug has been approved by health authorities in the USA,3 Mexico,4 South Africa5 and Canada.6 Feed supplementation with ZH by Zilmax®, the pioneer brand (Merck, Sharp & Dohme Corp. MSD, México) improves feed conversion rates and carcass yields at the expense of muscle mass,7 while reducing overall fat deposition.8 Increased protein synthesis in muscle fibres is due to altered transcriptional activity in the myosin heavy-chain isoforms.9 Nutrients that usually form fat are shifted to increase muscle synthesis.10 ZH is rapidly and completely absorbed within 12 h after oral administration with food. Elimination occurs through the urine (86 %) in a biphasic manner: first with a half-life of 15.3 ± 1.8 h, then a 98 % clearing of the drug in 48 h.11 The second phase is slower and allows detecting zilpaterol after a withdrawal time of 8 d; however, a withdrawal time of 2 to 4 d has been set as the standard for meat production.12
Recently, generic commercial ZH brands have become available in Mexico, but no information exists on their comparative efficacy regarding fattening efficiency and meat quality features, and no published information is available on ZH use in humid, tropical conditions, which are common in Mexican meat production. Moreover, most studies have been conducted on Bos taurus livestock, while cattle utilized for meat production in Mexico are mainly of the Bos indicus species.13 Thus, this study performed a non-inferiority assay to compare the pharmacodynamic responses of the reference ZH preparation (Zilmax® MSD) and a generic ZH brand (Zipamix® PiSA Agropecuaria S.A. de C.V., Mexico) on meat production and basic meat quality parameters, under a specific setting: Bos indicus cattle in humid tropical conditions.
Materials and methods
Procedures and bull management followed official Mexican regulations for animal care.15,16 The trial was approved by the Care Committee for Animal Use of Universidad Nacional Autónoma de México, based on the VICH GL9 guidelines for medical veterinary products.17 The trial was performed on bulls from a feedlot production located in the Tamuín region of San Luis Potosí, Mexico (22° 05›30.6» N, 98° 37’21.2” W). In this tropical region, temperatures are consistently high (ranging from 19.3 °C to 48.5 °C) with a mean of 25.5 °C throughout the year. Annual rainfall ranges from 800 to 1,500 mm and two weather seasons stand out: the drought season (late November through late May) and the rainy season (June to early November).18 This study was conducted during the rainy season, from August 10th, 2016 to September 14th, 2016. Humidity during this time ranged from 29 % to 96 %, and the temperature ranged from 33 °C to 49 °C. This region is classified as tropical savannah climate. Warm all year, with dry season (Aw), according to the climatic classification of Köppen19 with an average precipitation ranging from 1000 mm to 1400 mm/year.
Experimental design
Eight hundred nineteen zebu non-castrated bulls (approximately 75 % Bos indicus, 25 % Bos taurus), younger than 2 years old, weighing between 430 and 490 kg, and originating from southeastern Mexican farms (Chiapas and Veracruz) were selected for this trial. Upon selection, all bulls were treated with 200 µg/kg SC of ivermectin (Dectiver®, Lapisa, Mexico) and vaccinated against clostridial diseases (Ultrabac/Somubac®; Zoetis, Mexico). As in other related studies,20,21 an anabolic combination was implanted (i.e. Synovex-plus®; MSD) containing 200 mg of trenbolone acetate and 28 mg of oestradiol benzoate. Nine pens with similar shade, water and feed conditions were established with 90 animals each, and treatments were assigned randomly to each pen. The pens had mean surface areas of 1800 m2 with 600 m2 of shade and feeders of 40-m long, 70-cm wide and 60-cm deep. Automatic drinkers were located between pens. When the bulls arrived, they were quarantined to adapt to their new surroundings. Animal management and feeding followed the beef production unit’s standard procedures. The pens were constantly surveyed to isolate and, if necessary, discard bulls with evident signs of disease or injuries from the trial. All animals were subjected to an adaptation period of no less than two months before beginning the non-inferiority test.
Three groups with three replicates each were randomly established as follows: the control zilpaterol-free group (Cg); the ZHr group treated with the reference zilpaterol-HCl from Zilmax® (MSD) at a dose of 0.15 mg/kg/day, equivalent to an in-feed concentration of 6 ppm of zilpaterol HCl; and the ZHg group treated with the generic zilpaterol HCl from Zipamix® (PiSA-Agropecuaria, Mexico) with the same doses as the ZHr group. Both commercial brands contain 48 g of zilpaterol hydrochloride/kg of product, and the amount of commercial preparation added was 125 g/ton of feed in both cases.
Food was served twice daily (7:00 am and 1:00 pm) using Rotomix® automated trucks (International Trucks®, Laredo, TX, USA), with an integrated weighing machine to verify the quantity. In addition, a 3 % food excess was delivered based on previous food consumption records per body weight. Leftover food was removed, weighed and recorded daily. ZH mix homogeneity for both products was ensured by using micro-tracers (Micro-Tracers, Inc. San Francisco, EEUU). The premix was prepared weekly, and the feed was prepared with and without ZH twice daily. The medicated diet was provided for 30 days, and a withdrawal period of three days was Diet details are presented in Table 1. Before slaughter, towards the end of the trial (33 d), the ration was reduced by half for 12 hrs, and water was provided slaughter, towards the end of the trial (33 d), the ration was reduced by half for 12 hrs, and water was provided ad libitum. Thirty bulls per pen were then transported in trailers to the on-site slaughter house (Federal Inspected Slaughterhouse: TIF 470), at a distance of approximately 850 m. All animals were weighed individually just before slaughter, following Mexican regulations.23,24 To obtain the hot carcass weight (HCW), the animal’s head, viscera, legs and skin, were discarded. Carcasses were then cooled at 1 °C for 24 hrs to obtain the cold carcass weight (CCW).
Table 1 Dietary ingredients and chemical composition of the finishing diet as expressed in kg of each ingredient per ton of prepared food.
| Ingredient | Weight (kg/Ton) | % | Chemical composition | Before drying | Dried |
| Soja | 50 | 0.5 | ENm, Mcal/kg | 1.74 | 2.15 |
| DDG | 1400 | 14.0 | ENg, Mcal/kg | 1.18 | 1.50 |
| Molasess | 600 | 6.0 | Protein, % | 11.33 | 14.00 |
| Elit-f | 250 | 2.5 | Ash, % | 3.74 | 4.60 |
| Oil | 300 | 3.0 | Calcium, % | 0.68 | 0.85 |
| Corn | 6100 | 61.0 | Phosphor, % | 0.26 | 0.30 |
| Silo | 500 | 5.0 | FC, % | 5.32 | 6.60 |
| Straw | 800 | 8.0 | Ether extract, % | 5.33 | 6.60 |
| Humidity | 19.1 | Carb. Non-fibrous, % | 45.62 | 56.40 | 19.1 |
Meat production and meat quality
To analyse the meat twenty-four hrs after slaughter, 30 carcasses per treatment and per untreated animals, were randomly selected, using the online programme http://www.alazar.info/generador-de-numeros-aleatorios-sin-repeticion. After slaughter, the carcasses were stored in 9 refrigerators (three per treatment) and numbered consecutively. Two Longissimus dorsi muscle samples (cubes of 2.5 X 2.5 X 2.5 cm) were obtained per carcass at the 12th thoracic vertebra, one sample for the composition and proximal chemical analysis (PCA) and the other for sensory evaluation. Samples were vacuum-packed and shipped at 4 °C to the Meat Science Laboratory at the Facultad de Medicina Veterinaria y Zootecnia of Universidad Nacional Autónoma de México in Mexico City.
Subcutaneous fat and epimysium were removed and cleaned for the meat composition analysis. Samples were ground in a food processor and analysed for moisture content, intramuscular fat, protein quantity and ash following the method described by the Association of Official Analytical Chemists, AOAC.25 For sensory evaluation, the meat was cooked to an internal temperature of 70 °C, following the American Meat Science Association (AMSA) procedure.26 After removing the outer crust, cubes of approximately 2 x 2 x 2 cm were obtained and immediately served to 73 judges. To ensure unbiased observations, each judge received three samples without identification, low-salt biscuits were offered as flavour carriers, and water was available to rinse between samples. Judges were asked to indicate on a printed questionnaire their liking level for aroma, flavour, tenderness, juiciness, smoothness and overall acceptability of the meat, as well as on a seven-point hedonic scale (AMSA) in which 7 = I really liked it, and 1 = I really disliked it.
Statistical analysis
Treatments were initiated sequentially 24 hrs apart over 3 days to allow stepped work at the slaughter house. This arrangement allowed the study to be considered as a randomized block design. Each animal was used as the experimental unit for initial weight (i.e., weight at the beginning of the trial), final weight, weight gain, cold carcass and meat composition variables. For the food consumption and feed conversion variables, each pen was used as an experimental unit.
In this non-inferiority study, the generic product was expected to be at least 80 % as effective as the reference product. The null hypothesis established that the efficacy difference between treatments (ZHr and ZHg) was no larger than 20 %. Rejecting the null hypothesis leads to a non-inferiority conclusion,20 as follows:
Ho: T-S ≤ -δ ó T-S ≥ - δ
Ha: - δ < T - S < δ
δ = 20 %.22
Where δ is the non-inferiority margin, T is the test treatment and S is the standard active control treatment. For a non-inferiority trial, the generic product is expected to have at least an 80 % efficacy compared to the reference.27
Each animal was the experimental unit for the final weight, or average daily gain (ADG). For carcass characteristics such as cold carcass weight (CCW), cold carcass yield (CCY) and dressing % (carcass weight/live weight) x 100, the experimental unit was each carcass. For the meat composition (humidity, protein, fat and ash %) and sensory variables (aroma, flavour, tenderness, juiciness, smoothness and overall acceptability of the meat), each block meat sample was the experimental unit.
For the food consumption and feed conversion variables, each block was taken as treatment repetition (n = 3), and each pen was either treated with ZHr or ZHg or untreated (Cg). Treatments were initiated sequentially 24 hrs apart over 3 days. Thus, a randomised complete block design with a generalised linear model (GzLM) was applied.28 The assessed variables were initial body weight (BWi), final weight (BWf), total gain (TG), (ADG) kg/d average daily gain, dry mean intake (DMI), conversion kg:kg rate (G: F) and carcass characteristics of cold carcass weight (CCW), cold carcass yield (CCY), and dressing % (carcass weight/live weight) x 100. The linear link model was used to analyse the continuous variables, including treatment, block (confusion factor) and their interaction for BWf and TG. BWi was added as a confusion factor and BWf was added as a covariable to carcass characteristics. DMI, G:F and muscle composition percentages were analysed as a complete randomized design with a linear link for the GzLM. For the sensory variables, the percentages were quantified as positive responses (≥ 5) on the hedonic scale,26 and odd ratios (Wald 95 % CI) were calculated and evaluated by GzLM, using a fixed complete randomized design with a binomial probit link. Statistical support for the non-inferiority hypothesis was assessed by the Wald statistic.
All analyses were performed using IBM SPSS Statistics® version 21 for Windows® (IBM, Mexico S.A).29 Differences between ZHg, ZHr and the control group were considered significant if P was ≤ 0.05.30 The non-inferiority hypothesis was considered true if δ < 20 %. A power test (1-β) was performed with the G-Power programme.
Results and discussion
This study aimed to determine whether the pharmacodynamic effects of a generic ZH statistically differed from the reference ZH formulation, using a non-inferiority assay27 based on weight gain, secondary productive parameter variables, and the sensorial characteristics of the meat. Table 2 shows the Wald values for each model, their degrees of freedom (d.f.) and P-values. The observed results between ZHg and ZHr (Table 3) are statistically indistinguishable with a 95 % CI.
Table 2 Wald value for models tested with GzLM for production and carcass variables.
| Model components | ||||||
|---|---|---|---|---|---|---|
| Dependent variables | I | T | B Wald(d.f.) P-value | T*B | BWi | BWf |
| BWi | 2234279(1)a 0.0000001b | 0.688(2) 0.71 | 1685(2) 0.0001 | 2.8(4) 0.71 | - | - |
| BWf N = 819 | 5.1(1) 0.02 | 37.4(2) 0.0001 | 3,8(2) 0.15 | 19.7(4) 0.001 | 167.5(1) 0.00001 | - |
| ADG N = 819 | 5.1(1) 0.02 | 38.3(2) 0.0001 | 3.8(2) 0.15 | 19.9(4) 0.001 | 0.5(1) 0.46 | - |
| DMI N = 91 | 17560 0.00001 | 31.9 0.0001 | - | - | - | |
| G:F N = 9 | 2129(1) 0.0001 | 1.8(2) 0.410 | - | - | - | - |
| CCW N = 819 | 116.1(1) 0.0001 | 68.8(3) 0.0001 | 43.5(2) 0.0001 | 13.2(4) 0.01 | - | 420.8(1) 0.0001 |
| Dressing % N = 819 | 1807(1) 0.0001 | 69.6(2) 0.0001 | 57.8(2) 0.0001 | 12.7(4) 0.13 | 129.5 0.0001 | |
Production Characteristics: Initial Body Weight (BWi), Final weight (BWf), Average Daily Gain (ADG), Dry Mean Ingest (DMI), feed conversion: G:F (kg:kg), carcass characteristics: Cold Carcass Weight (CCW), Dressing % = (Carcass Weight/Live Weight) x 100; Model components: I: Intersection, T: treatment, B: Block, T*B (interaction between treatment and Block).
a,b Different literals mean significant differences between treatments (P < 0.01) in Bonferroni tests.
Table 3 Feedlot and carcass performance in bulls supplemented with zilpaterol hydrochloride (ZHg and ZHr).
| Treatment means1 | Wald CI 95 % 2 | Wald test Treatment 3 | |||||||
| Live Weight | Control N = 277 | ZHg N = 282 | ZHr N = 260 | Control | ZHg | ZHr | P | 1-β | Wald d.f. = 2 |
| Initial, kg | 465.74 a | 465.78 | 465.03 | 464.1, 466.2 | 464.2, 466.2 | 464.6, 466.8 | 0.710 | 0.78 | 0.69 |
| Final, kg | 509.02 a | 518.4 | 515.15 | 506.9, 511.2 | 516.3, 520.5 | 513.1, 517.2 | 0.001 | 0.92 | 37.40 |
| Total gain | 43.77 a | 53.16 | 49.91 | 41.5, 45.9 | 51.1, 55.2 | 47.8, 51.9 | 0.001 | 0.93 | 38.50 |
| ADG kg/d | 1.34 a | 1.61 | 1.51 | 1.26, 1.39 | 1.45, 1.57 | 1.54, 1.67 | 0.001 | 0.93 | 38.50 |
| DMI kg/d | 9.69 a N = 3 | 10.74 N = 3 | 10.41 N = 3 | 9.43, 9.9 | 10.48, 11.0 | 10.15, 10.7 | 0.001 | 0.73 | 28.11 |
| G:F kg/kg | 0.129 a N = 3 | 0.138 N = 3 | 0.136 N = 3 | 0.119, 0.13.8 | 0.128, 0.148 | 0.126, 0.146 | 0.410 | 0.67 | 1.79 |
| Cold Carcass Weight, kg | 308.39 a | 317.04 b | 314.49 b | 307.9, 311.0 | 315.8, 318.4 | 313.5, 316.1 | 0.0001 | 0.99 | 98.10 |
| Dressing % | 60.75 a | 62.25 b | 61.79 b | 60.7, 61.2 | 61.9, 62.4 | 61.5, 62.1 | 0.001 | 0.99 | 69.63 |
1. ZHg = zilpaterol hydrochloride from Zipamix® (Pisa Agropecuaria Mexico, Guadalajara, Mexico); ZHr = zilpaterol hydrochloride from Zilmax (MSD). ADG kg/d Average Daily Gain; DMI kg/d: Daily Mean Intake, G:F kg/kg food conversion.
2. 95 % Confidence Intervals with Wald statistic, d.f.: degrees of freedom.
3. Wald test for treatment factor; P: P-value; 1-β: Power of the test; Wald: Chi-square value.
a,b Different literals mean significant differences between treatments (P < 0.01) in Bonferroni tests.
An average weight gain of 7.76 kg was observed in the treated bulls compared with the control bulls (Table 3). The CCW performance showed a mean increase of 7.37 kg in the ZH-treated bulls, compared with the untreated animals (Cg). In contrast, feed conversion (G:F) did not significantly differ between treated and untreated bulls (note the 95 % Wald confidence intervals (CI), Table 3). These values confirm that ZH promotes growth. The cold carcass mean weight and carcass yield in the treatment groups, ZHg and ZHr, were statistically indistinguishable (95 % Wald CI). Given the large sample size, the test powers were between 0.66 and 0.99. Figure 1 shows the means and 95 % confidence intervals of the average daily gain, dressing and conversion rate (G:F; kg/kg). Note that for conversion rate, an overlap clearly exists between the latter groups, unlike for the other two variables in which the control groups did not overlap with the treated groups. ZHr-treated animals varied greatly in conversion rate. This was corrected in the GzLM model. Maximum likelihood was used with GzLM model to lower the adjusted mean variation (Figure 1).

Figure 1 Means and 95 % confidence intervals for dressing percentage, average daily gain and conversion rate (kg/kg) by treatment as follows: ZHg = zilpaterol hydrochloride from Zipamix® (Pisa Agropecuaria México, Guadalajara, Mexico); ZHr = zilpaterol hydrochloride from Zilmax (MERCK, SHARP & DOHME CORP. MSD Salud Animal México, Mexico City, Mexico).
Proximal chemical analysis data for the Longissimus dorsi muscle are presented in Table 4. The findings indicate that supplementing either commercial ZH brand does not modify the moisture, fat or ash content in the muscle (P = 0.32; Wald c2 test = 2.5; d.f.= 2), compared with the ZH-free animal meat (1-β = 0.63). The ZHr protein percentage was higher than that of the ZHg and control groups (24.16 and 23.52 %, respectively), and the test powers were between 0.6 and 0.82.
Table 4 Composition of the Longissimus dorsi muscle of bulls supplemented with zilpaterol hydrochloride (ZHg and ZHr)
| Treatment 1 | Wald 95 % IC | Wald Test 2 | |||||||
| Item (%) | Control N = 28* | ZHg N = 27* | ZHr N = 29* | Control | ZHg | ZHr | P (χ 2 ) (d.f. = 2) | 1-β | |
| Humidity | 71.26 | 70.85 | 71.39 | 70.78, 71.73 | 70.38, 71.31 | 70.9, 71.38 | 0.32(2.5) | 0.63 | |
| Protein | 23.52 a, b | 23.08 b | 24.16a | 22.9, 24.1 | 22.5, 23.6 | 23.6, 24.7 | 0.03(7.2) | 0.57 | |
| Fat | 4.36 b | 5.22 b | 3.58 a | 3.8, 4.9 | 4.7, 5.7 | 3.0, 4.1 | 0.001(18.4) | 0.75 | |
| Ash | 0.85 | 0.84 | 0.87 | 0.79, 0.89 | 0.80, 0.89 | 0.82, 0.91 | 0.792(0.46) | 0.82 | |
1. ZHg = zilpaterol hydrochloride from Zipamix® (Pisa Agropecuaria México, Guadalajara, Mexico); ZHr = zilpaterol hydrochloride from Zilmax (MSD).
a, b Different literals mean significant differences between treatments (P < 0.01) in Bonferroni tests.
2. P-value of overall test (Wald c2 statistic), degrees of freedom (d.f.), and power test (1-β).
* After slaughtering the animals, 30 carcasses were sampled for the meat analysis. However, some samples were not suitable for the analysis due to contamination.
Zilpaterol supplementation (ZHg and ZHr) did not modify the consumers’ scores for odour, taste and softness of the Longissimus dorsi muscle (P = 0.50; Wald χ2 test = 0.8; d.f. = 2). Table 5 summarizes these data. For these attributes, more than 65 % of consumers assigned scores ranging from 5 to 7 (i.e., I liked it lightly, and I really liked it) as observed in Figure 2. Meat juiciness from animals treated with either ZHg or ZHr presented lower scores (≤ 5) compared with the untreated animal meat (P = 0.046; χ2= 2; N = 219).
Table 5 Percentage of positive ratings* (≥ 5) for palatability traits of the bulls’ Longissimus dorsi muscle with zilpaterol hydrochloride (ZHg and ZHr); meat aged for 11 days.
| Treatment 1 | Odds ratio (Wald 95 % IC) | Wald Test 2 | |||||||
| Item | Control N = 73 | ZHg N = 73 | ZHr N = 73 | ZHg vs Cg | ZHr vs Cg | ZHr vs ZHg | P (χ 2 ) (d.f. = 1) | 1-β | |
| Aroma | 71.2 | 67.1 | 56.2 | 1.2 (0.6, 2.4) | 1.9 (0.97, 3.8) | 0.75 (0.49, 1.1) | 0.173 (3.8) | 0.6 | |
| Flavour | 75.3 | 68.5 | 65.8 | 1.4 (0.68, 2.9) | 1.6 (0.77, 3.3) | 1.10 (0.71,1.6) | 0.72 (1.7) | 0.74 | |
| Tenderness | 60.3 | 53.4 | 58.9 | 1.3 (0.68, 2,5) | 1.1 (0.55,2.0) | 0.80 (0.42, 1.5) | 0.50 (0.8) | 0.6 | |
| Juiciness | 65.8 a | 45.2 b | 56.2 b | 2.33 (1.2, 4.5) | 1.5 (0.77, 2.9) | 0.66 (0.77, 3.2) | 0.046 (6.2) | 0.5 | |
| Overall acceptability | 75.3 | 67.1 | 69.9 | 1.5 (0.73, 3.0) | 1.3 (0.63, 2.7) | 0.88 (0.44, 1.8) | 0.72 (1.2) | 0.74 | |
1. ZHg = zilpaterol hydrochloride from Zipamix® (Pisa Agropecuaria Mexico, Guadalajara, Mexico); ZHr = zilpaterol hydrochloride from Zilmax (MSD). a,b Different literals mean significant differences between treatments (P < 0.01) in Bonferroni tests.
2 Contrast between Zhg and ZHr of Odd ratios. P-value of overall test (Wald c2 statistic), degrees of freedom (d.f.), and power test (1-β).
* Consumer sensory panel ratings based on a hedonic 7-point scale (1 = disliked very much through 7 = liked very much).
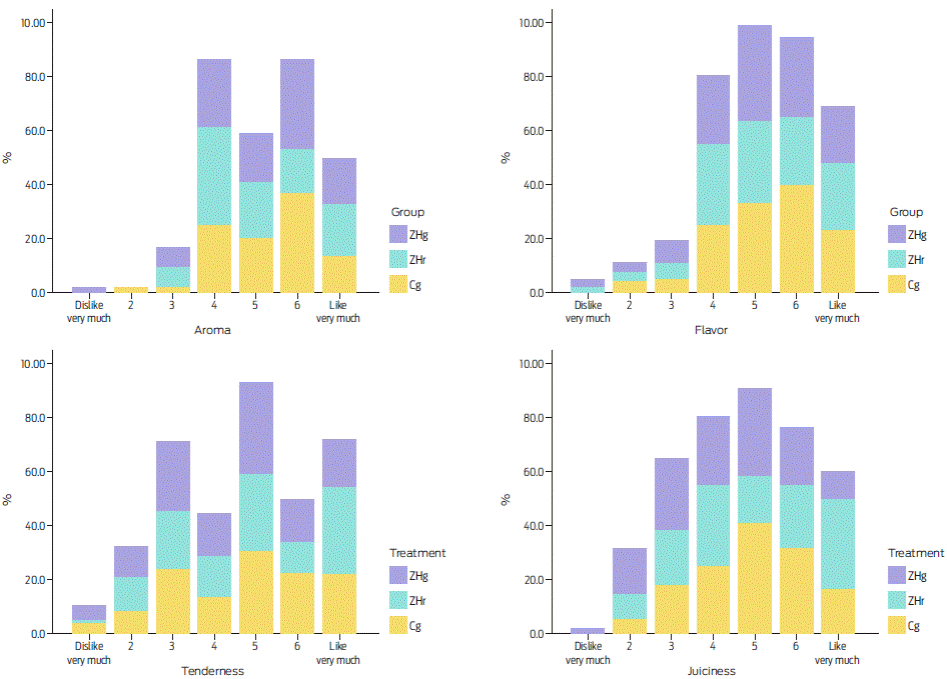
Figure 2 Sensorial hedonic scale percentages for aroma, flavour, tenderness and juiciness per treatment as follows: ZHg = zilpaterol hydrochloride from Zipamix® (Pisa Agropecuaria México, Guadalajara, Mexico); ZHr = zilpaterol hydrochloride from Zilmax (MSD).
For most variables assessed, differences between the groups treated with ZH and the control group were highly significant (P < 0.01). This demonstrates the efficacy of both ZH commercial brands. However, subtracting the mean values of the generic or the reference product from the untreated group reveals a higher difference favouring the generic commercial brand. This is illustrated in Table 3, where the difference between the final weight of the animals treated with the reference product and the control group is 6.13 kg. This value is 9.38 kg for the generic group; however, the difference between these groups was not statistically significant, indicating that the effectiveness of the ZHg treatment is not inferior to that of the reference product, ZHr. In other words, in all variables assessed between the ZHg and ZHr groups, the results confirmed the non-inferiority hypothesis. CCW and CCY mean values for the bulls treated with the generic ZH product did not differ from their corresponding values in the ZHr group; however, in this trial, the weight gain increments were 11.7 % in the ZHg group and 8.3 % in the ZHr group. Nevertheless, non-inferiority criteria require a minimum difference of at least 20 %.27
Predictably, both treatments were statistically superior to the control. The magnitude of improvement in final body weight (22 %), lies near the maximum value reported in the literature (25 %), using the reference brand ZH. 4,31-39 For example, weight gain coincides greatly with other studies conducted on Bos taurus bulls.34,40 Elam et al. treated Bos taurus bulls with 8.33 mg ZH/kg feed as dry matter for 30 days and obtained final weight increments of 9.3 kg compared with untreated bulls.42 These values are similar to those obtained in this trial for Bos indicus (i.e., final weight increments of 7.75 kg at a dose of 6 mg of generic ZH/kg feed). The mean final weight reported by Avendaño et al. using a generic ZH, was higher than the one achieved here21; however, the weight gain reported by these authors was similar to the corresponding value obtained during this trial (e.g., 77.80 vs. 53.16, respectively).
Differences in final weight (e.g., 528.83 kg vs 518.4 kg, respectively) could be due to differences in age, diet and climate. Most trials using the reference ZH brand were conducted in temperate/cold areas, which have predominant Bos taurus bulls33,42-44. After a thorough literature review, only two trials could be compared with the one described here. One occurred in Baja California, Mexico, which is a geographical area characterized by high environmental temperatures (annual mean of 25.3 °C with a range of 5 °C to 45 °C)18 and a BWh region (i.e., dry climate, with the driest season during winter and an average annual temperature higher than 18 °C), 21 corresponding to a desert-like scenario. When the study was conducted, the temperature oscillated approximately 20.3 °C with 57.6 % humidity,19 and zebu-type cattle were tested as well as 75/25 % Bos indicus/Bos taurus bulls. In that study, the total weight gain achieved with the generic brand was only 8.3 %, while in the present trial the same value reached 21.5 %. The second study was conducted in Yucatán, Mexico.20 This region has similar weather conditions to those in our trial (i.e., a humid climate ranging from 30.92 % to 69.08 % and high temperatures year-round, where the annual mean is 28 °C with a range of 16 °C to 40 °C).16 The region has been classified as Aw (i.e., humid tropical climate with a dry season during winter and at least one month with a monthly precipitation < 60 mm).19 The zebu bulls included in the research of Castellanos et al. 2006 were younger and lighter than those in our study, and no generic ZH brand was tested20; only the reference ZH from Zilmax® and an untreated control group were studied. These methodological differences hinder direct comparisons with the present trial; however, Castellanos et al. reported a final weight gain of only 1.05 % of the ZH-treated group compared with the control group (e.g., 48.6 kg vs 51 kg, respectively).20 Comparisons among studies are difficult as many factors could have influenced the results, such as diet digestibility, surface area of the provided shade, and weather conditions. Nevertheless, one issue appears to be clear in these three studies: the presence of particularly unfavourable climate conditions for optimal weight gain.45 Furthermore, competitive production variables were noticeably improved. For example, the mean temperature during this trial was 29.3 °C at 8:00 hours and 35.0 °C at 1:00 p.m., with relative humidity of 83.8 % and 53.2 %, respectively. Although the study was conducted during the rainy season, only three days experienced heavy rain. The bulls were supplemented with ZH only for 30 days, but this supplementation has been used up to 40 days.34 It appears that production variables are similar in either dosing scheme.7,20,21,32,34 The accepted meat withdrawal period in countries that have authorized ZH use for 30 to 40 days is 3 to 4 days.46,47 Within these dosing ranges, ZH supplementation appears to be economically profitable.19-48
In cattle, β-adrenergic agonist drugs are reported to modify the adipose tissue metabolism and decrease fat by increasing lipolysis and decreasing lipogenesis. In muscle tissue, these drugs increase protein by reducing its degradation and increasing protein synthesis.49 The obtained values from the proximal chemical analysis agree with the moisture, protein, fat and ash ranges for the Longissimus dorsi muscle of Mexican cattle,50,51 although the results found during this trial showed slightly higher protein and fat. In contrast, these data differ from values reported in the literature outside Mexico. Shook et al. 2002, evaluated the meat of British and British × Continental bulls treated with 8.3 mg ZH/kg feed as dry matter for 20 d.50 These authors found that the protein percent was higher in the meat from ZH-treated bulls, compared with untreated animals (23.41 vs 22.87 %), but fat deposition remained statistically indistinguishable. In contrast, Rathmann et al. 2009, found that ZH-treated animal meat had less fat and more muscle moisture and protein content compared with untreated animals.9 Holmer et al. 2009, mentioned that moisture was unaffected, but the amount of fat was lower in the measured muscles (Triceps brachii, Gluteus medius and Longissimus lumborum) from animals treated for 30 d with ZH.43 Hilton et al. 2009 observed a decrease in the fat percentage of the Longissimus lumborum muscle, but the protein and humidity percentages remained unaltered in ZH-supplemented bulls.52 Differences in the fat amount, protein content and humidity among the studies outside Mexico and the data gathered in this trial may be partly explained by the genetic contributions of Bos taurus and Bos indicus from each group of bulls, as Bos indicus have lower fat deposition rates than Bos taurus.54
Although consumers detected differences in the Longissimus dorsi muscle juiciness for both ZH treatments, the overall pleasing rating was unaffected (P = 0.046; Wald χ2 test = 6.2; d.f = 1). This agrees with the results of Garmyn36, who indicated that ZH does not modify Longissimus dorsi muscle taste from Holstein bulls compared with control samples. Despite sustained juiciness and general softness scores being affected, it is likely that these latter changes were related to the lesser amount of intramuscular fat in the ZH-treated animal meat.54 This effect was not demonstrated here; however, the reduced juiciness and general softness across all studies has been linked to muscle fibre hypertrophy.55,56
Finally, generic drug preparations fulfil a debatable but sometimes clear social benefit by reducing costs of reference-drug preparations, which occurred in this study. Additionally, ZH use dissuades clenbuterol use as an illegal agent to improve carcass yield, thus preventing human clenbuterol toxicity outbreaks.56 The lack of pharmacodynamic similarity is often seen as an argument for discrediting generic preparations; however, in this assay, carcass yield and meat quality from both ZH-tested preparations were indistinguishable.
Conclusions
Few countries have approved ZH use for the final phase of fattening beef cattle, and until now, non-inferiority data for generic preparations were lacking. One exception is an essay that estimated carcass quality when using a generic ZH brand (Grofactor® Virbac, Mexico)21; however, as environmental conditions are key factors for the well-being and fattening efficiency of bulls, that study failed to disclose details on the cattle housing such as shade surface or how the feed was served. Consequently, this is the first carefully structured trial to compare productive variables from a non-inferiority perspective, conducted under humid tropical conditions in cattle with a marked Bos indicus genotype.
Regarding meat quality, few differences (not statistically significant) were observed between reference and generic ZH preparations, and both improved meat production compared with the untreated animals. This evidence confirms the non-inferiority nature of the generic ZH preparation.











 nueva página del texto (beta)
nueva página del texto (beta)



