Introduction
The hemotropic rickettsia Anaplasma marginale causes bovine anaplasmosis and is transmitted biologically by ticks; however, different haematophagous Diptera (Tabanus sp., Haematobia irritans, Stomoxys calcitrans) can mechanically spread the rickettsia.1-3 In this context, anaplasmosis, together with bovine babesiosis, causes economic losses higher than 10 billion dollars worldwide.4 After transmission to bovines, the rickettsia multiplies in their erythrocytes.4 In the acute infection phase, the rickettsemia may reach 109 infected erythrocytes (IE)/mL of blood.5 The main clinical signs of anaplasmosis are anaemia, weight loss, and abortion, and death is common.5 Recovered bovines become long-lasting carriers (clinically healthy) in which the rickettsia is very difficult to detect in blood smears examined with the optical microscope.5,6
It is assumed that haematophagous flies mechanically participate in the transmission of A. marginale to the bovines, mainly during the acute phase of the infection in specific areas of the U.S.A.6 Furthermore, in some areas of Brazil7 and Costa Rica8 there is strong evidence that S. calcitrans actively participates in the mechanical transmission of A. marginale to cattle. In this connection, the minimal infectious dose (MID) of A. marginale-infected erythrocytes required for mechanical transmission is unknown. It has been suggested that it is in the range between the minimum of 1 IE and 100 IE.6 Approximations of the amount of blood transported on stable fly mouthparts range from 0.029 mL to 0.4 mL.9,10 At an MID of 100 IE, rickettsemias between 2.5 X 108 (or 108.4) IE/mL and 3.5 X 109 (or 109.5) IE/mL would be necessary for a single fly to transport 1 MID. In fact, outbreaks of bovine anaplasmosis have been documented in some geographical areas where there are no ticks.11
S. calcitrans, is one of the biting flies implicated in the mechanical transmission of A. marginale among bovines,12 but there are not published reports that demonstrate this assumption. On this basis, the aim of this study was to detect DNA from A. marginale in S. calcitrans caught at 6 meters near a bovine herd, housed in pens with cement floors, which has been maintained free of ticks for 40 years, and occasionally experienced some cases of anaplasmosis.
Materials and methods
Sampling site
The study was carried out at an animal facility located at Progreso, Jiutepec, Morelos State, Mexico. It stands at 18º 53´ N, 99º 10´ W, where the average warmest temperatures oscillate between 21.3 ºC (January) and 26.6 ºC (May), while the average coldest temperatures oscillate between 6.5 ºC (January) and 12.5 ºC (June). This facility houses a herd of 80 Aberdeen Angus cattle (65 females, 15 males) whose zootechnical function is meat production. Animals are housed in pens with concrete floors and they are fed with lucerne and a commercial supplement; and water is consumed ad libitum. This herd has sporadically experienced cases of anaplasmosis, and thus the bovines were examined every week to verify they have no ticks. There are a few farmers owning small numbers of cattle and horses in the vicinity.
Sampling flies
Groups of 15 S. calcitrans were caught without touching them, when the flies were resting after a blood meal on the walls close (6 meters) to the cattle pens. Flies were caught by using a new sterile 50 mL plastic tube, containing 3 mL of sterile distilled water. A tube was slowly and carefully placed on a fly resting on the wall, and then the tube lid was screwed for trapping the fly. Afterwards, the tube was shaken to wet the fly wings and avoid it to scape when the tube was opened again to trap another fly. This procedure was repeated until 15 flies were captured in each tube.
Catches were done in the morning and in the afternoon, twice a week (except for one catch in one week of September and one catch in one week of December) for a total of 12 catching days during September, October, November and December 2015. Flies were identified as Stomoxys calcitrans according to morphological characteristics (seven to eight mm in length and look like house flies).13 They can be distinguished by belly marks in the form of chessboard and by the mouthparts for skin piercing (Figure 1A).13 Only those stable flies showing evidence by visual inspection of having taken a blood meal were collected (Figure 1A - photograph obtained by Carlos R. Bautista-). It is worth mentioning that no other haematophagous dipterans were identified at the time of the study. Immediately after capture, batches of 15 flies were placed in an Eppendorf vial (2 mL), and then the specimens were anesthetized through cold to manipulate them until processed. Whole ground fly batches were stored at -70 °C and prepared as described by Scoles et al.6 DNA extraction was carried out using the UltraClean™ BloodSpin™ DNA Isolation kit (MO BIO Laboratories Inc., Carlsbad, CA, U.S.A.) following the manufacturer´s procedure.
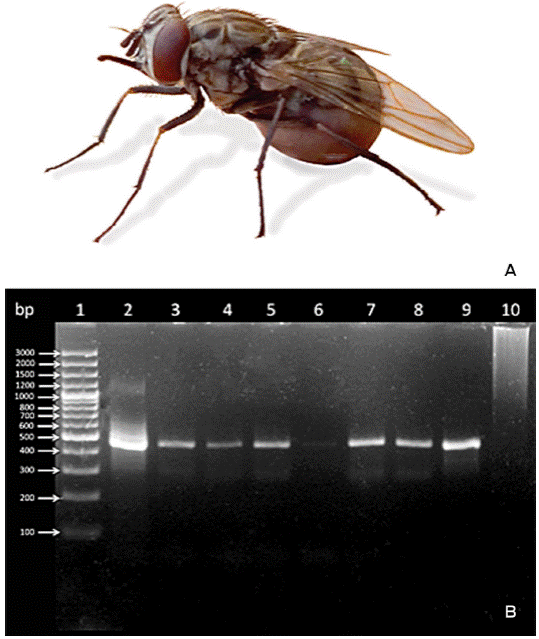
Figure 1 A. Example of a stable fly Stomoxys calcitrans caught in this study (photograph: Carlos R. Bautista). B. Agarose gel at 2 % stained with Ethidium bromide, which shows the amplification products of msp5 of A. marginale by nested PCR. Lane 1: markers; lane 2, positive control; lanes 3 to 9, Stomoxys calcitrans samples; lane 10, negative control. (Sample in well 6 is positive, although the line is faint).
Samples were tested for A. marginale DNA using a nested PCR (nPCR) that targets the amplification of a fragment from a single copy gene that encodes the outer major surface protein MSP5 from A. marginale. Reaction and amplification conditions were carried out as described by Torioni et al,13,14 using appropriate positive and negative controls. Sequences for the primers used were based on Torioni et al.13 and the expected amplicons are described in Table 1.
Table 1 Primers used for A. marginale DNA identification, based on the amplification of the Msp5 gene which codes for the major surface protein MSP5.
| Gene | Product | Primer sequence | Amplicon size (bp) |
| Msp5 | Major surface protein MSP5 | F: 5' GCATAGCCTCCCCCTCTTTC 3' | 548 |
| R: 5' TCCTCGCCTTGCCCCTCAGA 3' | |||
| F: 5' TACACGTGCCCTACCGACTTA 3' | 345 | ||
| R: 5' TCCTCGCCTTGCCCCTCAGA 3' |
Amplification conditions in a C1000 Touch™ Thermal cycler (BIO-RAD Laboratories, Inc., CA, U.S.A.) were: 35 cycles 95 °C for 3 minutes, followed by 35 cycles at 95 °C for 30 seconds, 35 cycles at 65 °C for 1 minute, and 35 cycles at 72 °C for 1 minute. A final extension step at 72 °C for 10 minutes was used, which was followed by 35 cycles at 4 ºC, at infinitum. The results of the PCR were analyzed in a 21 % agarose gel stained with Ethidium bromide. Samples were considered positive when amplification of a fragment corresponding to the expected amplicon size was visualized under ultraviolet light (Figure 1B).
Results and discussion
Seven out of 24 S. calcitrans groups were positive for A. marginale as detected by a nested PCR, and an expected amplicon size of 466 bp (Figure 1B). This represented 29.16 % of all the samples (Figure 2A). It was also observed that on two occasions, the S. calcitrans batches collected on the same day (morning and afternoon of October 22nd and of November 4th), were positive for A. marginale (16.66 %), while those S. calcitrans batches collected in the afternoon of October 1st, morning of November 6th, and afternoon of December 1st (25 %) were positive for the rickettsia (Figure 2B).
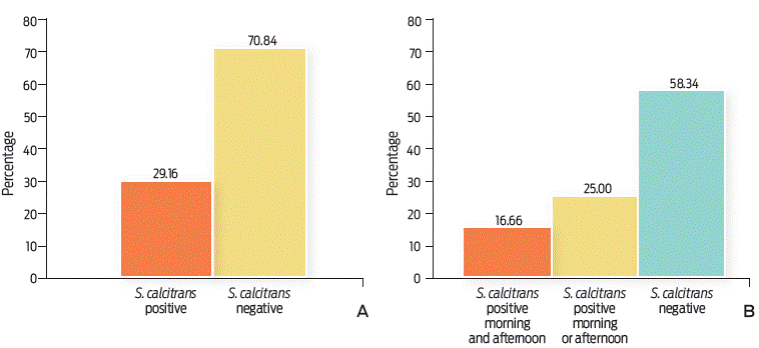
Figure 2 A. Percentage of S, calcitrans groups (15 flies / group) positive for A. marginale by nested PCR. Positive (n = 7), Negative (n = 17). B. Percentage of days of catching in which the S. calcitrans groups were positive for A. marginale. Samples positive in the morning and in the afternoon (n = 2: one day-October 22nd and one day-November 4th), samples positive in the morning or in the afternoon (n = 3: afternoon of October 1st, morning of November 6th, and afternoon of December 1st), Negative (n = 7).
The stable flies analyzed fed on tick-free animals of a bovine herd in an area (Morelos State, Mexico) considered as endemic of anaplasmosis.15 In an independent study carried out in bovines of the same herd (February 2017), it was observed that 15 (75 %) out of 20 animals were positive for anti-A. marginale antibodies by IFAT, (Pelaez-Flores A. 2017, personal communication). By comparison, in some dairy cattle herds seropositive for anti-Anaplasma marginale antibodies from Costa Rica, it has been observed that S. calcitrans is the only haematophagous parasite present,8 suggesting an active role in the mechanical transmission of A. marginale. Similarly, in recent and related studies, S. calcitrans has been associated with an outbreak of bovine anaplasmosis in Brazil.7
The results in the present study suggest that S. calcitrans mechanically transmitted A. marginale in bovines of a tick-free herd, and it is highly possible that contributed to the maintenance of the rickettsia in these animals. The recent finding of A. marginale DNA in a host other than cattle, like Myrmecophaga tridactyla (giant anteater),16 may expand the epidemiology of anaplasmosis, taking into account that S. calcitrans feeds from a variety of mammals,17,18 which may contribute to the mechanical transmission of A. marginale among different species. Under these circumstances, we hypothesize that A. marginale was mechanically introduced by biting flies into this bovine herd several years ago, and then the microorganism was mechanically transmitted by S. calcitrans (the most important haematophagous fly in this area) among the bovines. Thereafter, herd immunity19 was developed against A. marginale. However, some animals experience clinical anaplasmosis sporadically. In this context, a stable fly control program must be carried out the whole year in bovine industries in tropical and subtropical areas where anaplasmosis is endemic. It is advisable to take into account that in order to establish a control program it is necessary to generate information on the annual distribution of the ectoparasite in this particular area, because such information is not available.
Conclusions
Using a molecular test (nPCR) A. marginale DNA was detected in S. calcitrans caught next to a bovine herd, maintained free of ticks. This evidence indicates that the stable fly mechanically transmits this rickettsia, thereby circulating the pathogen among cattle in the absence of ticks. In further studies, it is suggested to test both flies (individually) and cattle at the same time for the presence of A. marginale DNA in order to better understand the epidemiology of anaplasmosis.











 nueva página del texto (beta)
nueva página del texto (beta)



